Sensory deprivation disrupts homeostatic regeneration of newly generated olfactory sensory neurons after injury in adult mice
- PMID: 25673857
- PMCID: PMC6605607
- DOI: 10.1523/JNEUROSCI.2484-14.2015
Sensory deprivation disrupts homeostatic regeneration of newly generated olfactory sensory neurons after injury in adult mice
Abstract
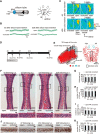
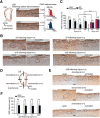
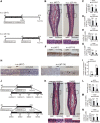
Although it is well known that injury induces the generation of a substantial number of new olfactory sensory neurons (OSNs) in the adult olfactory epithelium (OE), it is not well understood whether olfactory sensory input influences the survival and maturation of these injury-induced OSNs in adults. Here, we investigated whether olfactory sensory deprivation affected the dynamic incorporation of newly generated OSNs 3, 7, 14, and 28 d after injury in adult mice. Mice were unilaterally deprived of olfactory sensory input by inserting a silicone tube into their nostrils. Methimazole, an olfactotoxic drug, was also injected intraperitoneally to bilaterally ablate OSNs. The OE was restored to its preinjury condition with new OSNs by day 28. No significant differences in the numbers of olfactory marker protein-positive mature OSNs or apoptotic OSNs were observed between the deprived and nondeprived sides 0-7 d after injury. However, between days 7 and 28, the sensory-deprived side showed markedly fewer OSNs and mature OSNs, but more apoptotic OSNs, than the nondeprived side. Intrinsic functional imaging of the dorsal surface of the olfactory bulb at day 28 revealed that responses to odor stimulation were weaker in the deprived side compared with those in the nondeprived side. Furthermore, prevention of cell death in new neurons 7-14 d after injury promoted the recovery of the OE. These results indicate that, in the adult OE, sensory deprivation disrupts compensatory OSN regeneration after injury and that newly generated OSNs have a critical time window for sensory-input-dependent survival 7-14 d after injury.
Keywords: apoptosis; homeostatic regeneration; olfactory epithelium; olfactory sensory neuron; sensory deprivation.
Copyright © 2015 the authors 0270-6474/15/352657-17$15.00/0.
Figures






Similar articles
-
Insulin-Dependent Maturation of Newly Generated Olfactory Sensory Neurons after Injury.eNeuro. 2021 May 19;8(3):ENEURO.0168-21.2021. doi: 10.1523/ENEURO.0168-21.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33906971 Free PMC article.
-
Protective Effect of Insulin in Mouse Nasal Mucus Against Olfactory Epithelium Injury.Front Neural Circuits. 2021 Dec 23;15:803769. doi: 10.3389/fncir.2021.803769. eCollection 2021. Front Neural Circuits. 2021. PMID: 35002636 Free PMC article.
-
Differential copper-induced death and regeneration of olfactory sensory neuron populations and neurobehavioral function in larval zebrafish.Neurotoxicology. 2018 Dec;69:141-151. doi: 10.1016/j.neuro.2018.10.002. Epub 2018 Oct 4. Neurotoxicology. 2018. PMID: 30292653 Free PMC article.
-
Structures and functions of the normal and injured human olfactory epithelium.Front Neural Circuits. 2024 Jun 6;18:1406218. doi: 10.3389/fncir.2024.1406218. eCollection 2024. Front Neural Circuits. 2024. PMID: 38903957 Free PMC article. Review.
-
Regeneration and rewiring of rodent olfactory sensory neurons.Exp Neurol. 2017 Jan;287(Pt 3):395-408. doi: 10.1016/j.expneurol.2016.06.001. Epub 2016 Jun 3. Exp Neurol. 2017. PMID: 27264358 Review.
Cited by
-
Longer latency of sensory response to intravenous odor injection predicts olfactory neural disorder.Sci Rep. 2016 Oct 13;6:35361. doi: 10.1038/srep35361. Sci Rep. 2016. PMID: 27734933 Free PMC article.
-
Age-Related Olfactory Dysfunction: Epidemiology, Pathophysiology, and Clinical Management.Front Aging Neurosci. 2020 Jul 7;12:208. doi: 10.3389/fnagi.2020.00208. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32733233 Free PMC article. Review.
-
Pharmacologic modulation of nasal epithelium augments neural stem cell targeting of glioblastoma.Theranostics. 2019 Apr 6;9(7):2071-2083. doi: 10.7150/thno.29581. eCollection 2019. Theranostics. 2019. PMID: 31037157 Free PMC article.
-
Improving taste sensitivity in healthy adults using taste recall training: a randomized controlled trial.Sci Rep. 2022 Aug 16;12(1):13849. doi: 10.1038/s41598-022-18255-z. Sci Rep. 2022. PMID: 35974039 Free PMC article. Clinical Trial.
-
Low survival rate of young adult-born olfactory sensory neurons in the undamaged mouse olfactory epithelium.J Bioenerg Biomembr. 2019 Feb;51(1):41-51. doi: 10.1007/s10863-018-9774-8. Epub 2018 Oct 9. J Bioenerg Biomembr. 2019. PMID: 30302619 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
