Basal ganglia outputs map instantaneous position coordinates during behavior
- PMID: 25673860
- PMCID: PMC4323537
- DOI: 10.1523/JNEUROSCI.3245-14.2015
Basal ganglia outputs map instantaneous position coordinates during behavior
Abstract
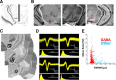
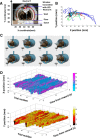
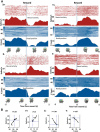
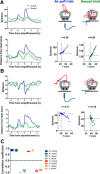
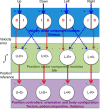
The basal ganglia (BG) are implicated in many movement disorders, yet how they contribute to movement remains unclear. Using wireless in vivo recording, we measured BG output from the substantia nigra pars reticulata (SNr) in mice while monitoring their movements with video tracking. The firing rate of most nigral neurons reflected Cartesian coordinates (either x- or y-coordinates) of the animal's head position during movement. The firing rates of SNr neurons are either positively or negatively correlated with the coordinates. Using an egocentric reference frame, four types of neurons can be classified: each type increases firing during movement in a particular direction (left, right, up, down), and decreases firing during movement in the opposite direction. Given the high correlation between the firing rate and the x and y components of the position vector, the movement trajectory can be reconstructed from neural activity. Our results therefore demonstrate a quantitative and continuous relationship between BG output and behavior. Thus, a steady BG output signal from the SNr (i.e., constant firing rate) is associated with the lack of overt movement, when a stable posture is maintained by structures downstream of the BG. Any change in SNr firing rate is associated with a change in position (i.e., movement). We hypothesize that the SNr output quantitatively determines the direction, velocity, and amplitude of voluntary movements. By changing the reference signals to downstream position control systems, the BG can produce transitions in body configurations and initiate actions.
Keywords: GABA; Parkinson's disease; basal ganglia; substantia nigra.
Copyright © 2015 the authors 0270-6474/15/352703-14$15.00/0.
Figures









References
-
- Bernstein N. The coordination and regulation of movements. Oxford: Pergamon; 1967.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
