A functional link between MT neurons and depth perception based on motion parallax
- PMID: 25673864
- PMCID: PMC4323539
- DOI: 10.1523/JNEUROSCI.3134-14.2015
A functional link between MT neurons and depth perception based on motion parallax
Abstract
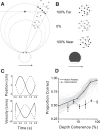
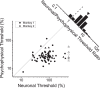
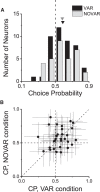
As an observer translates, objects lying at different distances from the observer have differential image motion on the retina (motion parallax). It is well established psychophysically that humans perceive depth rather precisely from motion parallax and that extraretinal signals may be used to correctly perceive the sign of depth (near vs far) when binocular and pictorial depth cues are absent or weak. However, the neural basis for this capacity remains poorly understood. We have shown previously that neurons in the macaque middle temporal (MT) area combine retinal image motion with smooth eye movement command signals to signal depth sign from motion parallax. However, those studies were performed in animals that were required simply to track a visual target, thus precluding direct comparisons between neural activity and behavior. Here, we examine the activity of MT neurons in rhesus monkeys that were trained to discriminate depth sign based on motion parallax, in the absence of binocular disparity and pictorial depth cues. We find that the most sensitive MT neurons approach behavioral sensitivity, whereas the average neuron is twofold to threefold less sensitive than the animal. We also find that MT responses are predictive of perceptual decisions (independent of the visual stimulus), consistent with a role for MT in providing sensory signals for this behavior. Our findings suggest that, in addition to its established roles in processing stereoscopic depth, area MT is well suited to contribute to perception of depth based on motion parallax.
Keywords: decision; depth; macaque; motion parallax; sensitivity.
Copyright © 2015 the authors 0270-6474/15/352766-12$15.00/0.
Figures






Similar articles
-
MT neurons combine visual motion with a smooth eye movement signal to code depth-sign from motion parallax.Neuron. 2009 Aug 27;63(4):523-32. doi: 10.1016/j.neuron.2009.07.029. Neuron. 2009. PMID: 19709633 Free PMC article.
-
Joint representation of depth from motion parallax and binocular disparity cues in macaque area MT.J Neurosci. 2013 Aug 28;33(35):14061-74, 14074a. doi: 10.1523/JNEUROSCI.0251-13.2013. J Neurosci. 2013. PMID: 23986242 Free PMC article.
-
Gain Modulation as a Mechanism for Coding Depth from Motion Parallax in Macaque Area MT.J Neurosci. 2017 Aug 23;37(34):8180-8197. doi: 10.1523/JNEUROSCI.0393-17.2017. Epub 2017 Jul 24. J Neurosci. 2017. PMID: 28739582 Free PMC article.
-
The neural basis of depth perception from motion parallax.Philos Trans R Soc Lond B Biol Sci. 2016 Jun 19;371(1697):20150256. doi: 10.1098/rstb.2015.0256. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27269599 Free PMC article. Review.
-
The role of areas MT and MST in coding of visual motion underlying the execution of smooth pursuit.Vision Res. 2008 Sep;48(20):2062-9. doi: 10.1016/j.visres.2008.04.015. Epub 2008 May 27. Vision Res. 2008. PMID: 18508104 Review.
Cited by
-
A neural mechanism for detecting object motion during self-motion.Elife. 2022 Jun 1;11:e74971. doi: 10.7554/eLife.74971. Elife. 2022. PMID: 35642599 Free PMC article.
-
A neural correlate of perceptual segmentation in macaque middle temporal cortical area.Nat Commun. 2022 Aug 24;13(1):4967. doi: 10.1038/s41467-022-32555-y. Nat Commun. 2022. PMID: 36002445 Free PMC article.
-
Dissociating representations of affect and motion in visual cortices.Cogn Affect Behav Neurosci. 2023 Oct;23(5):1322-1345. doi: 10.3758/s13415-023-01115-2. Epub 2023 Aug 1. Cogn Affect Behav Neurosci. 2023. PMID: 37526901 Free PMC article.
-
Do you hear what I see? How do early blind individuals experience object motion?Philos Trans R Soc Lond B Biol Sci. 2023 Jan 30;378(1869):20210460. doi: 10.1098/rstb.2021.0460. Epub 2022 Dec 13. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 36511418 Free PMC article. Review.
-
V1 neurons respond differently to object motion versus motion from eye movements.Nat Commun. 2015 Sep 15;6:8114. doi: 10.1038/ncomms9114. Nat Commun. 2015. PMID: 26370518 Free PMC article.
References
-
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol. 1984;51:16–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
