Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes
- PMID: 25673875
- PMCID: PMC4518445
- DOI: 10.1242/jcs.163303
Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes
Abstract
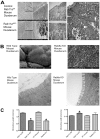
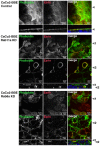
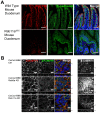
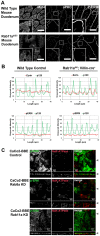
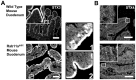
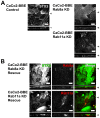
Rab11a is a key component of the apical recycling endosome that aids in the trafficking of proteins to the luminal surface in polarized epithelial cells. Utilizing conditional Rab11a-knockout specific to intestinal epithelial cells, and human colonic epithelial CaCo2-BBE cells with stable Rab11a knockdown, we examined the molecular and pathological impact of Rab11a deficiency on the establishment of apical cell polarity and microvillus morphogenesis. We demonstrate that loss of Rab11a induced alterations in enterocyte polarity, shortened microvillar length and affected the formation of microvilli along the lateral membranes. Rab11a deficiency in enterocytes altered the apical localization of syntaxin 3. These data affirm the role of Rab11a in apical membrane trafficking and the maintenance of apical microvilli in enterocytes.
Keywords: Enterocyte; Intestinal polarity; Microvilli; Rab11; Rab8; Syntaxin 3.
© 2015. Published by The Company of Biologists Ltd.
Figures


Similar articles
-
Trafficking Ion Transporters to the Apical Membrane of Polarized Intestinal Enterocytes.Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a027979. doi: 10.1101/cshperspect.a027979. Cold Spring Harb Perspect Biol. 2018. PMID: 28264818 Free PMC article. Review.
-
Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes.J Cell Sci. 2014 Mar 1;127(Pt 5):1007-17. doi: 10.1242/jcs.137273. Epub 2014 Jan 10. J Cell Sci. 2014. PMID: 24413175
-
Abnormal Rab11-Rab8-vesicles cluster in enterocytes of patients with microvillus inclusion disease.Traffic. 2017 Jul;18(7):453-464. doi: 10.1111/tra.12486. Epub 2017 May 17. Traffic. 2017. PMID: 28407399 Free PMC article.
-
Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy.J Cell Sci. 2017 Aug 1;130(15):2491-2505. doi: 10.1242/jcs.201897. Epub 2017 Jun 8. J Cell Sci. 2017. PMID: 28596241 Free PMC article.
-
RAB and RHO GTPases regulate intestinal crypt cell homeostasis and enterocyte function.Small GTPases. 2016 Apr 2;7(2):59-64. doi: 10.1080/21541248.2016.1159274. Epub 2016 May 4. Small GTPases. 2016. PMID: 27142493 Free PMC article. Review.
Cited by
-
Induction of lateral lumens through disruption of a monoleucine-based basolateral-sorting motif in betacellulin.J Cell Sci. 2015 Sep 15;128(18):3444-55. doi: 10.1242/jcs.170852. Epub 2015 Aug 13. J Cell Sci. 2015. PMID: 26272915 Free PMC article.
-
Oncogenic Pathways and Loss of the Rab11 GTPase Synergize To Alter Metabolism in Drosophila.Genetics. 2019 Aug;212(4):1227-1239. doi: 10.1534/genetics.119.302137. Epub 2019 Jun 18. Genetics. 2019. PMID: 31213502 Free PMC article.
-
Trafficking Ion Transporters to the Apical Membrane of Polarized Intestinal Enterocytes.Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a027979. doi: 10.1101/cshperspect.a027979. Cold Spring Harb Perspect Biol. 2018. PMID: 28264818 Free PMC article. Review.
-
Novel topography of the Rab11-effector interaction network within a ciliary membrane targeting complex.Small GTPases. 2015 Oct 2;6(4):165-73. doi: 10.1080/21541248.2015.1091539. Epub 2015 Sep 23. Small GTPases. 2015. PMID: 26399276 Free PMC article. Review.
-
Recycling endosomes.Curr Opin Cell Biol. 2015 Aug;35:117-22. doi: 10.1016/j.ceb.2015.04.018. Epub 2015 May 27. Curr Opin Cell Biol. 2015. PMID: 26022676 Free PMC article. Review.
References
-
- Calhoun BC, Lapierre LA, Chew CS, Goldenring JR. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am. J. Physiol. 1998;275:C163–C170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK048370/DK/NIDDK NIH HHS/United States
- R56 DK070856/DK/NIDDK NIH HHS/United States
- T32 DK007673/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK070856/DK/NIDDK NIH HHS/United States
- T32 CA119925/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- DK093809/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- CA178599/CA/NCI NIH HHS/United States
- DK085194/DK/NIDDK NIH HHS/United States
- DK102934/DK/NIDDK NIH HHS/United States
- R21 CA178599/CA/NCI NIH HHS/United States
- R01 DK102934/DK/NIDDK NIH HHS/United States
- DK48370/DK/NIDDK NIH HHS/United States
- R03 DK093809/DK/NIDDK NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- K01 DK085194/DK/NIDDK NIH HHS/United States
- R01 DK070856/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

