Paramagnetic centers in particulate formed from the oxidative pyrolysis of 1-methylnaphthalene in the presence of Fe(III)2O3 nanoparticles
- PMID: 25673882
- PMCID: PMC4321761
- DOI: 10.1016/j.combustflame.2013.06.025
Paramagnetic centers in particulate formed from the oxidative pyrolysis of 1-methylnaphthalene in the presence of Fe(III)2O3 nanoparticles
Abstract
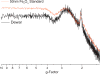
The identity of radical species associated with particulate formed from the oxidative pyrolysis of 1-methylnaphthalene (1-MN) was investigated using low temperature matrix isolation electron paramagnetic resonance spectroscopy (LTMI-EPR), a specialized technique that provided a method of sampling and analysis of the gas-phase paramagnetic components. A superimposed EPR signal was identified to be a mixture of organic radicals (carbon and oxygen-centered) and soot. The carbon-centered radicals were identified as a mixture of the resonance-stabilized indenyl, cyclopentadienyl, and naphthalene 1-methylene radicals through the theoretical simulation of the radical's hyperfine structure. Formation of these radical species was promoted by the addition of Fe(III)2O3 nanoparticles. Enhanced formation of resonance stabilized radicals from the addition of Fe(III)2O3 nanoparticles can account for the observed increased sooting tendency associated with Fe(III)2O3 nanoparticle addition.
Keywords: Annealing studies; Cryogenic trapping; EPR; Environmentally Persistent Free Radicals (EPFRs); Nanoparticles; Particulate.
Figures
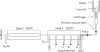
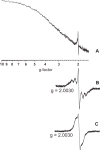
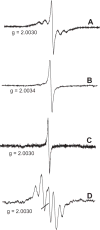




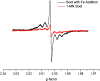
References
-
- Mansurov Z, Ongarbaev E, Tutkabaeva TT. Combust Flame. 1999;118(4):741–743.
-
- Akhter M, Chughtai A, Smith D. Appl Spectrosc. 1985;39(1):143–153.
-
- Chughtai A, Atteya M, Kim J, Konowalchuk B, Smith D. Carbon. 1998;36(11):1573–1589.
-
- Jones CC, Chughtai AR, Murugaverl B, Smith DM. Carbon. 2004;42(12–13):2471–2484.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
