Effects of interferon-γ knockdown on vaccine-induced immunity against Marek's disease in chickens
- PMID: 25673902
- PMCID: PMC4283228
Effects of interferon-γ knockdown on vaccine-induced immunity against Marek's disease in chickens
Abstract
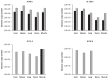
Interferon (IFN)-γ has been shown to be associated with immunity to Marek's disease virus (MDV). The overall objective of this study was to investigate the causal relationship between IFN-γ and vaccine-conferred immunity against MDV in chickens. To this end, 3 small interfering RNAs (siRNAs) targeting chicken IFN-γ, which had previously been shown to reduce IFN-γ expression in vitro, and a control siRNA were selected to generate recombinant avian adeno-associated virus (rAAAV) expressing short-hairpin small interfering RNAs (shRNAs). An MDV challenge trial was then conducted: chickens were vaccinated with herpesvirus of turkey (HVT), administered the rAAAV expressing shRNA, and then challenged with MDV. Tumors were observed in 4 out of 10 birds that were vaccinated with HVT and challenged but did not receive any rAAAV, 5 out of 9 birds that were administered the rAAAV containing IFN-γ shRNA, and 2 out of 10 birds that were administered a control enhanced green fluorescent protein siRNA. There was no significant difference in MDV genome load in the feather follicle epithelium of the birds that were cotreated with the vaccine and the rAAAV compared with the vaccinated MDV-infected birds. These results suggest that AAAV-based vectors can be used for the delivery of shRNA into chicken cells. However, administration of the rAAAV expressing shRNA targeting chicken IFN-γ did not seem to fully abrogate vaccine-induced protection.
Il a été démontré que l’interféron (INF)-γ est associé à l’immunité contre le virus de la maladie de Marek (VMM). L’objectif général de la présente étude était d’examiner la relation causale entre l’IFN-γ et l’immunité conférée par le vaccin contre le VMM chez les poulets. Pour y parvenir, trois petits ARN interférant (siARN) ciblant l’IFN-γ, et qui avaient préalablement été montré comme étant capable de réduire l’expression in vitro de l’IFN-γ, et un siARN témoin furent choisis afin de générer du virus adéno-associé aviaire recombinant (rAAAV) exprimant de courtes boucles de siRNA (shRNA). Un essai d’infection par VMM fut alors réalisé : des poulets furent vaccinés avec de l’herpèsvirus de dinde (HVT), reçurent le rAAAV exprimant les shRNA, et par la suite challengés avec le VMM. Des tumeurs furent observées chez 4 des 10 poulets qui avaient été vacciné avec HVT et challengés mais qui n’avaient pas reçu aucun rAAAV, 5 des 9 oiseaux qui avaient reçu le rAAAV contenant l’IFN-γ avec les shRNA, et 2 des 10 oiseaux témoins qui avaient reçu un siRNA qui augmentait la protéine fluorescente verte. Il n’y avait aucune différence significative dans la charge de génome de VMM dans l’épithélium du follicule des plumes des oiseaux qui avaient été co-traités avec le vaccin et le rAAAV comparativement aux oiseaux non-vaccinés avec MMV et infectés. Ces résultats suggèrent que les vecteurs à base d’AAAV peuvent être utilisés pour la livraison de shRNA dans les cellules des oiseaux. Toutefois, l’administration de rAAAV exprimant des shRNA ciblant l’IFN-γ des oiseaux n’a pas semblé complètement abrogé la protection induite par le vaccin.(Traduit par Docteur Serge Messier).
Figures
Similar articles
-
Interferon-γ influences immunity elicited by vaccines against very virulent Marek's disease virus.Antiviral Res. 2011 Jun;90(3):218-26. doi: 10.1016/j.antiviral.2011.04.001. Epub 2011 Apr 8. Antiviral Res. 2011. PMID: 21501630
-
Construction of Recombinant HVT Expressing PmpD, and Immunological Evaluation against Chlamydia psittaci and Marek's Disease Virus.PLoS One. 2015 Apr 20;10(4):e0124992. doi: 10.1371/journal.pone.0124992. eCollection 2015. PLoS One. 2015. PMID: 25893439 Free PMC article.
-
Phenotypic characterization of gamma delta (γδ) T cells in chickens infected with or vaccinated against Marek's disease virus.Virology. 2022 Mar;568:115-125. doi: 10.1016/j.virol.2022.01.012. Epub 2022 Jan 25. Virology. 2022. PMID: 35152043
-
Pathogenesis of Marek's disease (MD) and possible mechanisms of immunity induced by MD vaccine.J Vet Med Sci. 1998 Jan;60(1):1-8. doi: 10.1292/jvms.60.1. J Vet Med Sci. 1998. PMID: 9492353 Review.
-
Vaccinal control of Marek's disease: current challenges, and future strategies to maximize protection.Vet Immunol Immunopathol. 2006 Jul 15;112(1-2):78-86. doi: 10.1016/j.vetimm.2006.03.014. Epub 2006 May 8. Vet Immunol Immunopathol. 2006. PMID: 16682084 Review.
Cited by
-
A Marek's Disease Virus Messenger RNA-Based Vaccine Modulates Local and Systemic Immune Responses in Chickens.Viruses. 2024 Jul 18;16(7):1156. doi: 10.3390/v16071156. Viruses. 2024. PMID: 39066318 Free PMC article.
-
Differential Virus-Specific IFN-Gamma Producing T Cell Responses to Marek's Disease Virus in Chickens With B19 and B21 MHC Haplotypes.Front Immunol. 2022 Jan 13;12:784359. doi: 10.3389/fimmu.2021.784359. eCollection 2021. Front Immunol. 2022. PMID: 35095857 Free PMC article.
-
Temporal Dynamics of Purinergic Receptor Expression in the Lungs of Marek's Disease (MD) Virus-Infected Chickens Resistant or Susceptible to MD.Viruses. 2024 Jul 14;16(7):1130. doi: 10.3390/v16071130. Viruses. 2024. PMID: 39066292 Free PMC article.
-
The effects of in ovo administration of encapsulated Toll-like receptor 21 ligand as an adjuvant with Marek's disease vaccine.Sci Rep. 2018 Nov 6;8(1):16370. doi: 10.1038/s41598-018-34760-6. Sci Rep. 2018. PMID: 30401976 Free PMC article.
-
Mardivirus Infection and Persistence in Feathers of a Chicken Model Harboring a Local Autoimmune Response.Microorganisms. 2020 Oct 20;8(10):1613. doi: 10.3390/microorganisms8101613. Microorganisms. 2020. PMID: 33092272 Free PMC article.
References
-
- Calnek BW. Pathogenesis of Marek’s disease virus infection. Curr Top Microbiol Immunol. 2001;255:25–55. - PubMed
-
- Haq K, Brisbin JT, Thanthrige-Don N, Heidari M, Sharif S. Transcriptome and proteome profiling of host responses to Marek’s disease virus in chickens. Vet Immunol Immunopathol. 2010;138:292–302. - PubMed
-
- Abdul-Careem MF, Hunter BD, Parvizi P, Haghighi HR, Thanthrige-Don N, Sharif S. Cytokine gene expression patterns associated with immunization against Marek’s disease in chickens. Vaccine. 2007;25:424–432. - PubMed
-
- Haq K, Elawadli I, Parvizi P, Mallick AI, Behboudi S, Sharif S. Interferon-gamma influences immunity elicited by vaccines against very virulent Marek’s disease virus. Antiviral Res. 2011;90:218–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
