Development of a murine ocular posterior segment explant culture for the study of intravitreous vector delivery
- PMID: 25673906
- PMCID: PMC4283231
Development of a murine ocular posterior segment explant culture for the study of intravitreous vector delivery
Abstract
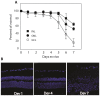
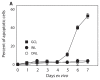
The objective of this study was to develop a murine retinal/choroidal/scleral explant culture system to facilitate the intravitreous delivery of vectors. Posterior segment explants from adult mice of 2 different age groups (4 wk and 15 wk) were cultured in serum-free medium for variable time periods. Tissue viability was assessed by gross morphology, cell survival quantification, activated caspase-3 expression, and immunohistochemistry. To model ocular gene therapy, explants were exposed to varying transducing units of a lentiviral vector expressing the gene for green fluorescent protein for 48 h. Explant retinal cells remained viable for approximately 1 wk, although the ganglion cell layer developed apoptosis between 4 and 7 d. Following vector infusion into the posterior segment cups, viral transduction was noted in multiple retinal layers in both age groups. An age of donor mouse influence was noted and older mice did not transduce as well as younger mice. This explant offers an easily managed posterior segment ocular culture with minimum disturbance of the tissue, and may be useful for investigating methods of enhancing retinal gene therapy under controlled conditions.
L’objectif de la présente étude était de développer un système murin de culture d’explant de rétine/choroïde/sclérotique afin de faciliter la livraison intra-vitréenne de vecteurs. Des explants de segments postérieurs provenant de souris adultes de deux groupes d’âge différents (4 sem et 15 sem) furent cultivés dans un milieu sans sérum pour des périodes de temps variables. La viabilité tissulaire fut évaluée par morphologie macroscopique, quantification de la survie cellulaire, expression de la caspase-3 activée, et immunohistochimie. Afin d’imiter la thérapie génique oculaire, les explants furent exposés pendant 48 h à des unités de transduction variables d’un vecteur lentiviral exprimant le gène pour la protéine fluorescente verte. Les explants de cellules de la rétine sont demeurés viables pour environ 1 sem, bien que dans la couche de cellules ganglionnaires on nota le développement de l’apoptose entre 4 à 7 j. Suite à l’infusion de vecteur dans le segment postérieur, la transduction virale fut notée dans plusieurs couches rétiniennes des animaux des deux groupes d’âge. Une influence de l’âge de la souris donneuse fut notée et chez les souris plus âgées la transduction ne se faisait pas aussi bien que chez les jeunes souris. Ce modèle d’explant permet la gestion facile de culture de segment oculaire postérieur avec un minimum de dérangement du tissu, et pourrait être utile pour étudier des méthodes visant à augmenter la thérapie génique sous conditions contrôlées.(Traduit par Docteur Serge Messier).
Figures




Similar articles
-
Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model.Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3503-12. doi: 10.1167/iovs.07-1601. Epub 2008 Apr 11. Invest Ophthalmol Vis Sci. 2008. PMID: 18408186
-
Sustained transduction of ocular cells with a bovine immunodeficiency viral vector.Hum Gene Ther. 2002 Jul 20;13(11):1305-16. doi: 10.1089/104303402760128531. Hum Gene Ther. 2002. PMID: 12162813
-
Improvement of Photoreceptor Targeting via Intravitreal Delivery in Mouse and Human Retina Using Combinatory rAAV2 Capsid Mutant Vectors.Invest Ophthalmol Vis Sci. 2017 Dec 1;58(14):6429-6439. doi: 10.1167/iovs.17-22281. Invest Ophthalmol Vis Sci. 2017. PMID: 29260200 Free PMC article.
-
Jugular Vein Injection of High-Titer Lentiviral Vectors Does Not Transduce the Aorta-Brief Report.Arterioscler Thromb Vasc Biol. 2021 Mar;41(3):1149-1155. doi: 10.1161/ATVBAHA.120.315125. Epub 2020 Dec 10. Arterioscler Thromb Vasc Biol. 2021. PMID: 33297756 Free PMC article.
-
[Developments in gene delivery vectors for ocular gene therapy].Med Sci (Paris). 2015 May;31(5):529-37. doi: 10.1051/medsci/20153105015. Epub 2015 Jun 9. Med Sci (Paris). 2015. PMID: 26059304 Review. French.
Cited by
-
Brain-derived neurotrophic factor prevents dendritic retraction of adult mouse retinal ganglion cells.Eur J Neurosci. 2016 Aug;44(3):2028-39. doi: 10.1111/ejn.13295. Epub 2016 Jul 19. Eur J Neurosci. 2016. PMID: 27285957 Free PMC article.
References
-
- Zhang SS, Fu XY, Barnstable CJ. Tissue culture studies of retinal development. Methods. 2002;28:439–447. - PubMed
-
- Beier M, Franke A, Paunel-Gorgulu AN, Scheerer N, Dunker N. Transforming growth factor beta mediates apoptosis in the ganglion cell layer during all programmed cell death periods of the developing murine retina. Neuro Res. 2006;56:193–203. - PubMed
-
- Caffe AR, Soderpalm A, van Veen T. Photoreceptor-specific protein expression of mouse retina in organ culture and retardation of rd degeneration in vitro by a combination of basic fibroblast and nerve growth factors. Curr Eye Res. 1993;12:719–726. - PubMed
-
- Engelsberg K, Ehinger B, Wasselius J, Johansson K. Apoptotic cell death and microglial cell responses in cultured rat retina. Graefes Arch Clin Exp Ophthalmol. 2004;242:229–239. - PubMed
-
- Feigenspan A, Bormann J, Wassle H. Organotypic slice culture of the mammalian retina. Vis Neurosci. 1993;10:203–217. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
