Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: in vitro and in vivo study
- PMID: 25673985
- PMCID: PMC4321605
- DOI: 10.2147/IJN.S78675
Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: in vitro and in vivo study
Abstract
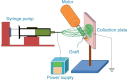
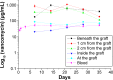
This study describes novel biodegradable, drug-eluting nanofiber-loaded vascular prosthetic grafts that provide local and sustained delivery of vancomycin to surrounding tissues. Biodegradable nanofibers were prepared by first dissolving poly(D,L)-lactide-co-glycolide and vancomycin in 1,1,1,3,3,3-hexafluoro-2-propanol. The solution was then electrospun into nanofibers onto the surface of vascular prostheses. The in vitro release rates of the pharmaceutical from the nanofiber-loaded prostheses was characterized using an elution method and a high-performance liquid chromatography assay. Experimental results indicated that the drug-eluting prosthetic grafts released high concentrations of vancomycin in vitro (well above the minimum inhibitory concentration) for more than 30 days. In addition, the in vivo release behavior of the drug-eluting grafts implanted in the subcutaneous pocket of rabbits was also documented. The drug-eluting grafts developed in this work have potential applications in assisting the treatment of vascular prosthesis infection and resisting reinfection when an infected graft is to be exchanged.
Keywords: drug-eluting prosthetic graft; release characteristics; vascular prosthesis infection.
Figures




Similar articles
-
Sustained local delivery of high-concentration vancomycin from a hybrid biodegradable, antibiotic-eluting, nanofiber-loaded endovascular prosthesis for treatment of mycotic aortic aneurysms.J Vasc Surg. 2018 Aug;68(2):597-606. doi: 10.1016/j.jvs.2017.07.142. Epub 2017 Oct 21. J Vasc Surg. 2018. PMID: 29066243
-
Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: in vitro and in vivo studies.Int J Nanomedicine. 2014 Sep 12;9:4347-55. doi: 10.2147/IJN.S66526. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25246790 Free PMC article.
-
A biodegradable antibiotic-eluting PLGA nanofiber-loaded deproteinized bone for treatment of infected rabbit bone defects.J Biomater Appl. 2016 Aug;31(2):241-9. doi: 10.1177/0885328216654424. Epub 2016 Jun 10. J Biomater Appl. 2016. PMID: 27288462
-
Dual delivery of active antibactericidal agents and bone morphogenetic protein at sustainable high concentrations using biodegradable sheath-core-structured drug-eluting nanofibers.Int J Nanomedicine. 2016 Aug 17;11:3927-37. doi: 10.2147/IJN.S107250. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27574423 Free PMC article.
-
Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery.Int J Nanomedicine. 2014;9:311-26. doi: 10.2147/IJN.S51258. Epub 2014 Jan 6. Int J Nanomedicine. 2014. PMID: 24421640 Free PMC article.
Cited by
-
User input in iterative design for prevention product development: leveraging interdisciplinary methods to optimize effectiveness.Drug Deliv Transl Res. 2017 Oct;7(5):761-770. doi: 10.1007/s13346-017-0397-0. Drug Deliv Transl Res. 2017. PMID: 28653286 Free PMC article.
-
Vascular Graft Impregnation with Antibiotics: The Influence of High Concentrations of Rifampin, Vancomycin, Daptomycin, and Bacteriophage Endolysin HY-133 on Viability of Vascular Cells.Med Sci Monit Basic Res. 2017 Jun 27;23:250-257. doi: 10.12659/msmbr.902879. Med Sci Monit Basic Res. 2017. PMID: 28652563 Free PMC article.
-
Evolution of drug-eluting biomedical implants for sustained drug delivery.Eur J Pharm Biopharm. 2021 Feb;159:21-35. doi: 10.1016/j.ejpb.2020.12.005. Epub 2020 Dec 16. Eur J Pharm Biopharm. 2021. PMID: 33338604 Free PMC article. Review.
-
Synergistic Effect of Co-Delivering Ciprofloxacin and Tetracycline Hydrochloride for Promoted Wound Healing by Utilizing Coaxial PCL/Gelatin Nanofiber Membrane.Int J Mol Sci. 2022 Feb 8;23(3):1895. doi: 10.3390/ijms23031895. Int J Mol Sci. 2022. PMID: 35163814 Free PMC article.
-
Targeted Delivery of Bioactive Molecules for Vascular Intervention and Tissue Engineering.Front Pharmacol. 2018 Nov 21;9:1329. doi: 10.3389/fphar.2018.01329. eCollection 2018. Front Pharmacol. 2018. PMID: 30519186 Free PMC article. Review.
References
-
- Ryan SV, Calligaro KD, Scharff J, Dougherty MJ. Management of infected prosthetic dialysis arteriovenous grafts. J Vasc Surg. 2004;39(1):73–78. - PubMed
-
- Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. - PubMed
-
- Peng CW, Tan SG. Polyurethane grafts: a viable alternative for dialysis arteriovenous access? Asian Cardiovasc Thorac Ann. 2003;11(4):314–318. - PubMed
-
- Englesbe MJ, Al-Holou WN, Moyer AT, et al. Single center review of femoral arteriovenous grafts for hemodialysis. World J Surg. 2006;30(2):171–175. - PubMed
-
- Schutte WP, Helmer SD, Salazar L, Smith JL. Surgical treatment of infected prosthetic dialysis arteriovenous grafts: total versus partial graft excision. Am J Surg. 2007;193(3):385–388. discussion 388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

