The effect size, study design, and development experience in commercially sponsored studies for new drug applications in approved drugs
- PMID: 25674470
- PMCID: PMC4320132
- DOI: 10.1186/2193-1801-3-740
The effect size, study design, and development experience in commercially sponsored studies for new drug applications in approved drugs
Abstract
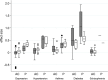
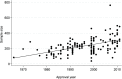
Pharmaceutical companies incorporate different features into the trials for new drug applications (NDAs) to render them efficient, making use of their experience. The objective of this analysis was to examine the associations between outcome and features related to study design and clinical development experience in commercially sponsored clinical trials. We collected data of phase 2 and phase 3 trials of all the drugs that obtained approval for depression, schizophrenia, asthma, hypertension, and diabetes in Japan from 1970 to 2011. In total, 145 trials from 90 test drugs were eligible for our study. We calculated the effect size, the standard mean of differences between test drug and comparator therapeutic effects, as the objective variable for use in our analysis. A linear mixed effect model with nested and crossed random effects was used in the analysis including variety of therapeutic area, test drugs and clinical trials. The analysis showed that trial features including sample size, subjective endpoints and active comparator of the same mode of action were negatively associated with effect size. In addition, sponsors' domestic clinical development experience with similar drugs seemed to have a positive association, but prior development experience in foreign countries did not. The accumulation of skills and knowledge within sponsors, and accumulated experience in domestic professionals who implement clinical trials under study contracts with sponsors would be of great importance for yielding clear outcomes. This study provides additional evidence with respect to possible sizes and directions of the influence of study design features that must be considered when planning and implementing trials for new drug applications, and when retrospectively comparing outcomes from trials with different designs and environments.
Keywords: Development experience; Effect size; New drug application; Randomized controlled trial; Study design.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

