The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission
- PMID: 25675099
- PMCID: PMC4347858
- DOI: 10.1371/journal.ppat.1004672
The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission
Abstract
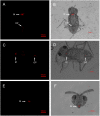
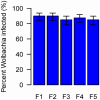
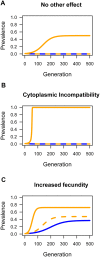
Facultative bacterial endosymbionts are associated with many arthropods and are primarily transmitted vertically from mother to offspring. However, phylogenetic affiliations suggest that horizontal transmission must also occur. Such horizontal transfer can have important biological and agricultural consequences when endosymbionts increase host fitness. So far horizontal transmission is considered rare and has been difficult to document. Here, we use fluorescence in situ hybridization (FISH) and multi locus sequence typing (MLST) to reveal a potentially common pathway of horizontal transmission of endosymbionts via parasitoids of insects. We illustrate that the mouthparts and ovipositors of an aphelinid parasitoid become contaminated with Wolbachia when this wasp feeds on or probes Wolbachia-infected Bemisia tabaci AsiaII7, and non-lethal probing of uninfected B. tabaci AsiaII7 nymphs by parasitoids carrying Wolbachia resulted in newly and stably infected B. tabaci matrilines. After they were exposed to infected whitefly, the parasitoids were able to transmit Wolbachia efficiently for the following 48 h. Whitefly infected with Wolbachia by parasitoids had increased survival and reduced development times. Overall, our study provides evidence for the horizontal transmission of Wolbachia between insect hosts by parasitic wasps, and the enhanced survival and reproductive abilities of insect hosts may adversely affect biological control programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Buchner P (1965) Endosymbiosis of animals with plant microorganisms. New York: John Wiley and Sons Interscience Press; pp. 332–338.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

