Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy
- PMID: 25675143
- PMCID: PMC4367511
- DOI: 10.1089/humc.2014.154
Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy
Abstract
Large animal models of genetic diseases are rapidly becoming integral to biomedical research as technologies to manipulate the mammalian genome improve. The creation of cystic fibrosis (CF) ferrets and pigs is an example of such progress in animal modeling, with the disease phenotypes in the ferret and pig models more reflective of human CF disease than mouse models. The ferret and pig CF models also provide unique opportunities to develop and assess the effectiveness of gene and cell therapies to treat affected organs. In this review, we examine the organ disease phenotypes in these new CF models and the opportunities to test gene therapies at various stages of disease progression in affected organs. We then discuss the progress in developing recombinant replication-defective adenoviral, adeno-associated viral, and lentiviral vectors to target genes to the lung and pancreas in ferrets and pigs, the two most affected organs in CF. Through this review, we hope to convey the potential of these new animal models for developing CF gene and cell therapies.
Figures
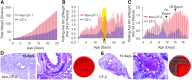

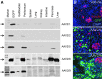
References
-
- Riordan JR, Rommens JM, Kerem B, et al. . Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073 - PubMed
-
- Rommens JM, Iannuzzi MC, Kerem B, et al. . Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 1989;245:1059–1065 - PubMed
-
- Kerem B, Rommens JM, Buchanan JA, et al. . Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–1080 - PubMed
-
- Rich DP, Anderson MP, Gregory RJ, et al. . Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990;347:358–363 - PubMed
-
- Tang L, Fatehi M, and Linsdell P. Mechanism of direct bicarbonate transport by the CFTR anion channel. J Cyst Fibros 2009;8:115–121 - PubMed
Publication types
MeSH terms
Grants and funding
- P01 HL091842/HL/NHLBI NIH HHS/United States
- DK096518/DK/NIDDK NIH HHS/United States
- R24 DK096518/DK/NIDDK NIH HHS/United States
- P01 HL051670/HL/NHLBI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- HL123482/HL/NHLBI NIH HHS/United States
- HL108902/HL/NHLBI NIH HHS/United States
- Canadian Institutes of Health Research/Canada
- R01 HL108902/HL/NHLBI NIH HHS/United States
- DK054759/DK/NIDDK NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- R24 HL123482/HL/NHLBI NIH HHS/United States
- DK092284/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

