Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death
- PMID: 25675296
- PMCID: PMC4669795
- DOI: 10.1038/cddis.2015.16
Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death
Abstract
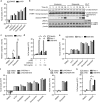
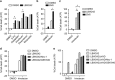
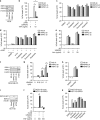
Apoptosis is a key mechanism for metazoans to eliminate unwanted cells. Resistance to apoptosis is a hallmark of many cancer cells and a major roadblock to traditional chemotherapy. Recent evidence indicates that inhibition of caspase-dependent apoptosis sensitizes many cancer cells to a form of non-apoptotic cell death termed necroptosis. This has led to widespread interest in exploring necroptosis as an alternative strategy for anti-cancer therapy. Here we show that in human colon cancer tissues, the expression of the essential necroptosis adaptors receptor interacting protein kinase (RIPK)1 and RIPK3 is significantly decreased compared with adjacent normal colon tissues. The expression of RIPK1 and RIPK3 was suppressed by hypoxia, but not by epigenetic DNA modification. To explore the role of necroptosis in chemotherapy-induced cell death, we used inhibitors of RIPK1 or RIPK3 kinase activity, and modulated their expression in colon cancer cell lines using short hairpin RNAs. We found that RIPK1 and RIPK3 were largely dispensable for classical chemotherapy-induced cell death. Caspase inhibitor and/or second mitochondria-derived activator of caspase mimetic, which sensitize cells to RIPK1- and RIPK3-dependent necroptosis downstream of tumor necrosis factor receptor-like death receptors, also did not alter the response of cancer cells to chemotherapeutic agents. In contrast to the RIPKs, we found that cathepsins are partially responsible for doxorubicin or etoposide-induced cell death. Taken together, these results indicate that traditional chemotherapeutic agents are not efficient inducers of necroptosis and that more potent pathway-specific drugs are required to fully harness the power of necroptosis in anti-cancer therapy.
Conflict of interest statement
JB and PJ are employees of GlaxoSmithKline. All other authors declare no potential conflict of interest.
Figures



References
-
- 1Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. - PubMed
-
- 2Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev 2012; 38: 589–598. - PubMed
-
- 3Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31: 51–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

