Quantification of cell-free mSHOX2 Plasma DNA for therapy monitoring in advanced stage non-small cell (NSCLC) and small-cell lung cancer (SCLC) patients
- PMID: 25675432
- PMCID: PMC4326280
- DOI: 10.1371/journal.pone.0118195
Quantification of cell-free mSHOX2 Plasma DNA for therapy monitoring in advanced stage non-small cell (NSCLC) and small-cell lung cancer (SCLC) patients
Abstract
Purpose: Most patients suffering from advanced lung cancer die within a few months. To exploit new therapy regimens we need better methods for the assessment of a therapy response.
Material and methods: In a pilot study we prospectively enrolled 36 patients with advanced NSCLC and SCLC (34 stage IV, 2 stage IIIB) of whom 34 received standard platinum-based chemo/radiotherapy and two were treated with a tyrosine kinase inhibitor. We measured the levels of extracellular methylated SHOX2 DNA (mSHOX2) in plasma before and during therapy until re-staging. The mSHOX2 analysis was blinded with respect to the clinical data making it an observational study.
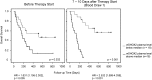
Results: According to the re-staging of 31 first-line patients, 19 patients were classified as non-responders while 12 patients were in the responder group. We observed a tight correlation between radiological data and the change of plasma mSHOX2 level as the equivalent for a therapy response. A ROC analysis showed a high discriminatory power for both patient groups already one week after therapy start (AUC 0.844). Additionally, a Kaplan-Meier and Cox Proportional Hazards analyses revealed a strong relationship between survival and plasma mSHOX2 value p ≤ 0.001 (hazard ratio 11.08) providing some evidence for mSHOX2 also being a predictive marker.
Conclusion: The longitudinal measurement of extracellular plasma mSHOX2 DNA yields information about the response to cytotoxic treatment and allows an early assessment of treatment response for lung cancer patients. If confirmed in a larger study this would be a valuable tool for selecting and guiding a cytotoxic treatment.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

