Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice
- PMID: 25675483
- PMCID: PMC4345564
- DOI: 10.1073/pnas.1414930112
Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice
Abstract
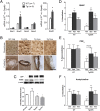
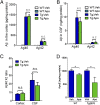
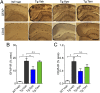
Cerebral amyloid angiopathy (CAA) is characterized by deposition of amyloid β peptide (Aβ) within walls of cerebral arteries and is an important cause of intracerebral hemorrhage, ischemic stroke, and cognitive dysfunction in elderly patients with and without Alzheimer's Disease (AD). NADPH oxidase-derived oxidative stress plays a key role in soluble Aβ-induced vessel dysfunction, but the mechanisms by which insoluble Aβ in the form of CAA causes cerebrovascular (CV) dysfunction are not clear. Here, we demonstrate evidence that reactive oxygen species (ROS) and, in particular, NADPH oxidase-derived ROS are a key mediator of CAA-induced CV deficits. First, the NADPH oxidase inhibitor, apocynin, and the nonspecific ROS scavenger, tempol, are shown to reduce oxidative stress and improve CV reactivity in aged Tg2576 mice. Second, the observed improvement in CV function is attributed both to a reduction in CAA formation and a decrease in CAA-induced vasomotor impairment. Third, anti-ROS therapy attenuates CAA-related microhemorrhage. A potential mechanism by which ROS contribute to CAA pathogenesis is also identified because apocynin substantially reduces expression levels of ApoE-a factor known to promote CAA formation. In total, these data indicate that ROS are a key contributor to CAA formation, CAA-induced vessel dysfunction, and CAA-related microhemorrhage. Thus, ROS and, in particular, NADPH oxidase-derived ROS are a promising therapeutic target for patients with CAA and AD.
Keywords: Alzheimer's disease; NADPH oxidase; cerebral amyloid angiopathy; reactive oxygen species; vasomotor dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition.J Neurosci. 2008 Dec 10;28(50):13542-50. doi: 10.1523/JNEUROSCI.4686-08.2008. J Neurosci. 2008. PMID: 19074028 Free PMC article.
-
Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress.Mol Neurodegener. 2023 Oct 3;18(1):73. doi: 10.1186/s13024-023-00660-1. Mol Neurodegener. 2023. PMID: 37789345 Free PMC article.
-
Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice.J Neurosci. 2003 Aug 27;23(21):7889-96. doi: 10.1523/JNEUROSCI.23-21-07889.2003. J Neurosci. 2003. PMID: 12944519 Free PMC article.
-
Cerebral amyloid angiopathy: pathogenesis and effects on the ageing and Alzheimer brain.Neurol Res. 2003 Sep;25(6):611-6. doi: 10.1179/016164103101202057. Neurol Res. 2003. PMID: 14503015 Review.
-
Cerebral amyloid angiopathy-related hemorrhages.Neurol Sci. 2008 Sep;29 Suppl 2:S260-3. doi: 10.1007/s10072-008-0957-7. Neurol Sci. 2008. PMID: 18690512 Review.
Cited by
-
Apolipoprotein E and Sex Bias in Cerebrovascular Aging of Men and Mice.Trends Neurosci. 2016 Sep;39(9):625-637. doi: 10.1016/j.tins.2016.07.002. Epub 2016 Aug 18. Trends Neurosci. 2016. PMID: 27546867 Free PMC article. Review.
-
Possible Predictors of Involuntary Weight Loss in Patients with Alzheimer's Disease.PLoS One. 2016 Jun 27;11(6):e0157384. doi: 10.1371/journal.pone.0157384. eCollection 2016. PLoS One. 2016. PMID: 27347878 Free PMC article.
-
Efficacy and Safety of Direct Oral Anticoagulant for Treatment of Atrial Fibrillation in Cerebral Amyloid Angiopathy.Cureus. 2020 Aug 30;12(8):e10143. doi: 10.7759/cureus.10143. Cureus. 2020. PMID: 32884877 Free PMC article.
-
Dysfunction of the Blood-brain Barrier in Cerebral Microbleeds: from Bedside to Bench.Aging Dis. 2021 Dec 1;12(8):1898-1919. doi: 10.14336/AD.2021.0514. eCollection 2021 Dec. Aging Dis. 2021. PMID: 34881076 Free PMC article. Review.
-
Small Vessel Disease-Related Dementia: An Invalid Neurovascular Coupling?Int J Mol Sci. 2020 Feb 7;21(3):1095. doi: 10.3390/ijms21031095. Int J Mol Sci. 2020. PMID: 32046035 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

