Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt
- PMID: 25675494
- PMCID: PMC4345602
- DOI: 10.1073/pnas.1417432112
Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt
Abstract
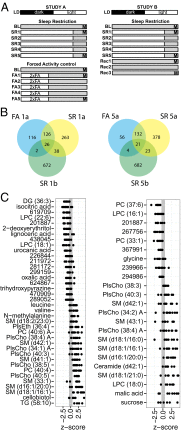
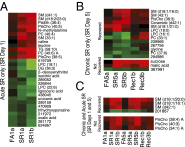
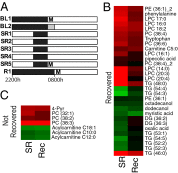
Sleep is an essential biological process that is thought to have a critical role in metabolic regulation. In humans, reduced sleep duration has been associated with risk for metabolic disorders, including weight gain, diabetes, obesity, and cardiovascular disease. However, our understanding of the molecular mechanisms underlying effects of sleep loss is only in its nascent stages. In this study we used rat and human models to simulate modern-day conditions of restricted sleep and addressed cross-species consequences via comprehensive metabolite profiling. Serum from sleep-restricted rats was analyzed using polar and nonpolar methods in two independent datasets (n = 10 per study, 3,380 measured features, 407 identified). A total of 38 features were changed across independent experiments, with the majority classified as lipids (18 from 28 identified). In a parallel human study, 92 metabolites were identified as potentially significant, with the majority also classified as lipids (32 of 37 identified). Intriguingly, two metabolites, oxalic acid and diacylglycerol 36:3, were robustly and quantitatively reduced in both species following sleep restriction, and recovered to near baseline levels after sleep restriction (P < 0.05, false-discovery rate < 0.2). Elevated phospholipids were also noted after sleep restriction in both species, as well as metabolites associated with an oxidizing environment. In addition, polar metabolites reflective of neurotransmitters, vitamin B3, and gut metabolism were elevated in sleep-restricted humans. These results are consistent with induction of peroxisome proliferator-activated receptors and disruptions of the circadian clock. The findings provide a potential link between known pathologies of reduced sleep duration and metabolic dysfunction, and potential biomarkers for sleep loss.
Keywords: lipidomics; mass spectrometry; metabolomics; sleep deprivation; sleep restriction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: A population-based study. J Clin Sleep Med. 2005;1(4):357–363. - PubMed
-
- Di Milia L, Vandelanotte C, Duncan MJ. The association between short sleep and obesity after controlling for demographic, lifestyle, work and health related factors. Sleep Med. 2013;14(4):319–323. - PubMed
-
- Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2014;3(1):52–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

