Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice
- PMID: 25675503
- PMCID: PMC4345598
- DOI: 10.1073/pnas.1419921112
Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice
Abstract
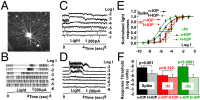
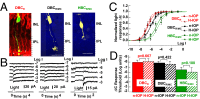
Glaucoma is the second leading cause of blindness in the United States and the world, characterized by progressive degeneration of the optic nerve and retinal ganglion cells (RGCs). Glaucoma patients exhibit an early diffuse loss of retinal sensitivity followed by focal loss of RGCs in sectored patterns. Recent evidence has suggested that this early sensitivity loss may be associated with dysfunctions in the inner retina, but detailed cellular and synaptic mechanisms underlying such sensitivity changes are largely unknown. In this study, we use whole-cell voltage-clamp techniques to analyze light responses of individual bipolar cells (BCs), AII amacrine cells (AIIACs), and ON and sustained OFF alpha-ganglion cells (ONαGCs and sOFFαGCs) in dark-adapted mouse retinas with elevated intraocular pressure (IOP). We present evidence showing that elevated IOP suppresses the rod ON BC inputs to AIIACs, resulting in less sensitive AIIACs, which alter AIIAC inputs to ONαGCs via the AIIAC→cone ON BC→ONαGC pathway, resulting in lower ONαGC sensitivity. The altered AIIAC response also reduces sOFFαGC sensitivity via the AIIAC→sOFFαGC chemical synapses. These sensitivity decreases in αGCs and AIIACs were found in mice with elevated IOP for 3-7 wk, a stage when little RGC or optic nerve degeneration was observed. Our finding that elevated IOP alters neuronal function in the inner retina before irreversible structural damage occurs provides useful information for developing new diagnostic tools and treatments for glaucoma in human patients.
Keywords: AII amacrine cells; bipolar cells; ganglion cells; glaucoma; intraocular pressure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gordon MO, et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720, discussion 829–830. - PubMed
-
- Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: Clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86(10):1803–1830. - PubMed
-
- Lee BL, et al. The Glaucoma Symptom Scale. A brief index of glaucoma-specific symptoms. Arch Ophthalmol. 1998;116(7):861–866. - PubMed
-
- Quigley HA. Ganglion cell death in glaucoma: Pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995;23(2):85–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

