Localizing a gate in CFTR
- PMID: 25675504
- PMCID: PMC4345560
- DOI: 10.1073/pnas.1420676112
Localizing a gate in CFTR
Abstract
Experimental and computational studies have painted a picture of the chloride permeation pathway in cystic fibrosis transmembrane conductance regulator (CFTR) as a short narrow tunnel flanked by wider inner and outer vestibules. Although these studies also identified a number of transmembrane segments (TMs) as pore-lining, the exact location of CFTR's gate(s) remains unknown. Here, using a channel-permeant probe, [Au(CN)2](-), we provide evidence that CFTR bears a gate that coincides with the predicted narrow section of the pore defined as residues 338-341 in TM6. Specifically, cysteines introduced cytoplasmic to the narrow region (i.e., positions 344 in TM6 and 1148 in TM12) can be modified by intracellular [Au(CN)2](-) in both open and closed states, corroborating the conclusion that the internal vestibule does not harbor a gate. However, cysteines engineered to positions external to the presumed narrow region (e.g., 334, 335, and 337 in TM6) are all nonreactive toward cytoplasmic [Au(CN)2](-) in the absence of ATP, whereas they can be better accessed by extracellular [Au(CN)2](-) when the open probability is markedly reduced by introducing a second mutation, G1349D. As [Au(CN)2](-) and chloride ions share the same permeation pathway, these results imply a gate is situated between amino acid residues 337 and 344 along TM6, encompassing the very segment that may also serve as the selectivity filter for CFTR. The unique position of a gate in the middle of the ion translocation pathway diverges from those seen in ATP-binding cassette (ABC) transporters and thus distinguishes CFTR from other members of the ABC transporter family.
Keywords: ABC transporters; anion channels; cystic fibrosis; gating.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

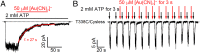

Similar articles
-
Spatial positioning of CFTR's pore-lining residues affirms an asymmetrical contribution of transmembrane segments to the anion permeation pathway.J Gen Physiol. 2016 May;147(5):407-22. doi: 10.1085/jgp.201511557. J Gen Physiol. 2016. PMID: 27114613 Free PMC article.
-
The Fifth Transmembrane Segment of Cystic Fibrosis Transmembrane Conductance Regulator Contributes to Its Anion Permeation Pathway.Biochemistry. 2015 Jun 23;54(24):3839-50. doi: 10.1021/acs.biochem.5b00427. Epub 2015 Jun 10. Biochemistry. 2015. PMID: 26024338 Free PMC article.
-
Cysteine scanning of CFTR's first transmembrane segment reveals its plausible roles in gating and permeation.Biophys J. 2013 Feb 19;104(4):786-97. doi: 10.1016/j.bpj.2012.12.048. Biophys J. 2013. PMID: 23442957 Free PMC article.
-
Conformational changes opening and closing the CFTR chloride channel: insights from cysteine scanning mutagenesis.Biochem Cell Biol. 2014 Dec;92(6):481-8. doi: 10.1139/bcb-2014-0038. Epub 2014 Sep 12. Biochem Cell Biol. 2014. PMID: 25367045 Review.
-
CFTR: what's it like inside the pore?J Exp Zool A Comp Exp Biol. 2003 Nov 1;300(1):69-75. doi: 10.1002/jez.a.10311. J Exp Zool A Comp Exp Biol. 2003. PMID: 14598388 Review.
Cited by
-
Fluorescence assay for simultaneous quantification of CFTR ion-channel function and plasma membrane proximity.J Biol Chem. 2020 Dec 4;295(49):16529-16544. doi: 10.1074/jbc.RA120.014061. Epub 2020 Sep 15. J Biol Chem. 2020. PMID: 32934006 Free PMC article.
-
Spatial positioning of CFTR's pore-lining residues affirms an asymmetrical contribution of transmembrane segments to the anion permeation pathway.J Gen Physiol. 2016 May;147(5):407-22. doi: 10.1085/jgp.201511557. J Gen Physiol. 2016. PMID: 27114613 Free PMC article.
-
Structural mechanisms for defective CFTR gating caused by the Q1412X mutation, a severe Class VI pathogenic mutation in cystic fibrosis.J Physiol. 2019 Jan;597(2):543-560. doi: 10.1113/JP277042. Epub 2018 Dec 2. J Physiol. 2019. PMID: 30408177 Free PMC article.
-
Structural mechanisms of CFTR function and dysfunction.J Gen Physiol. 2018 Apr 2;150(4):539-570. doi: 10.1085/jgp.201711946. Epub 2018 Mar 26. J Gen Physiol. 2018. PMID: 29581173 Free PMC article. Review.
-
Of muscle modulation and the CFTR gate.J Gen Physiol. 2015 Apr;145(4):255. doi: 10.1085/jgp.201511391. J Gen Physiol. 2015. PMID: 25825167 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

