Facile synthesis of novel graphene sponge for high performance capacitive deionization
- PMID: 25675835
- PMCID: PMC4327409
- DOI: 10.1038/srep08458
Facile synthesis of novel graphene sponge for high performance capacitive deionization
Abstract
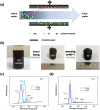
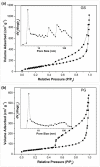
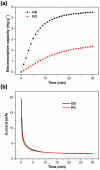
Capacitive deionization (CDI) is an effective desalination technique offering an appropriate route to obtain clean water. In order to obtain excellent CDI performance, a rationally designed structure of electrode materials has been an urgent need for CDI application. In this work, a novel graphene sponge (GS) was proposed as CDI electrode for the first time. The GS was fabricated via directly freeze-drying graphene oxide solution followed by annealing in nitrogen atmosphere. The morphology, structure and electrochemical performance of GS were characterized by scanning electron microscopy, Raman spectroscopy, nitrogen adsorption-desorption, X-ray photoelectron spectroscopy, cyclic voltammetry and electrochemical impedance spectroscopy. The electrosorption performance of GS in NaCl solution was studied and compared with pristine graphene (PG). The results show that due to the unique 3D interconnected porous structure, large accessible surface area and low charge transfer resistance, GS electrode exhibits an ultrahigh electrosorption capacity of 14.9 mg g(-1) when the initial NaCl concentration is ~500 mg L(-1), which is about 3.2 times of that of PG (4.64 mg g(-1)), and to our knowledge, it should be the highest value reported for graphene electrodes in similar experimental conditions by now. These results indicate that GS should be a promising candidate for CDI electrode.
Figures





References
-
- Yang Z. Y. et al. Sponge-templated preparation of high surface area graphene with ultrahigh capacitive deionization performance. Adv. Funct. Mater. 24, 3917–3925 (2014).
-
- Yin H. et al. Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv. Mater. 25, 6270–6276 (2013). - PubMed
-
- Wen X., Zhang D., Yan T., Zhang J. & Shi L. Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization. J. Mater. Chem. A 1, 12334–12344 (2013).
-
- Garcia-Quismondo E. et al. New testing procedures of a capacitive deionization reactor. Phys. Chem. Chem. Phys. 15, 7648–7656 (2013). - PubMed
-
- Hojati-Talemi P., Zou L., Fabretto M. & Short R. D. Using oxygen plasma treatment to improve the performance of electrodes for capacitive water deionization. Electrochim. Acta 106, 494–499 (2013).
LinkOut - more resources
Full Text Sources
Other Literature Sources

