Understanding of molecular mechanisms in natural killer cell therapy
- PMID: 25676064
- PMCID: PMC4346487
- DOI: 10.1038/emm.2014.114
Understanding of molecular mechanisms in natural killer cell therapy
Abstract
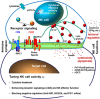
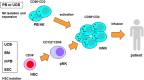
Cancer cells and the immune system are closely related and thus influence each other. Although immune cells can suppress cancer cell growth, cancer cells can evade immune cell attack via immune escape mechanisms. Natural killer (NK) cells kill cancer cells by secreting perforins and granzymes. Upon contact with cancer cells, NK cells form immune synapses to deliver the lethal hit. Mature NK cells are differentiated from hematopoietic stem cells in the bone marrow. They move to lymph nodes, where they are activated through interactions with dendritic cells. Interleukin-15 (IL-15) is a key molecule that activates mature NK cells. The adoptive transfer of NK cells to treat incurable cancer is an attractive approach. A certain number of activated NK cells are required for adoptive NK cell therapy. To prepare these NK cells, mature NK cells can be amplified to obtain sufficient numbers of NK cells. Alternatively, NK cells can be differentiated and amplified from hematopoietic stem cells. In addition, the selection of donors is important to achieve maximal efficacy. In this review, we discuss the overall procedures and strategies of NK cell therapy against cancer.
Figures

References
-
- Narni-Mancinelli E, Ugolini S, Vivier E. Tuning the threshold of natural killer cell responses. Curr Opin Immunol. 2013;25:53–58. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

