Synonymous codon usage affects the expression of wild type and F508del CFTR
- PMID: 25676312
- PMCID: PMC4355305
- DOI: 10.1016/j.jmb.2015.02.003
Synonymous codon usage affects the expression of wild type and F508del CFTR
Abstract
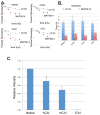
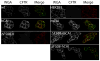
The cystic fibrosis transmembrane conductance regulator (CFTR) is an anion channel composed of 1480 amino acids. The major mutation responsible for cystic fibrosis results in loss of amino acid residue, F508 (F508del). Loss of F508 in CFTR alters the folding pathway resulting in endoplasmic-reticulum-associated degradation. This study investigates the role of synonymous codon in the expression of CFTR and CFTR F508del in human HEK293 cells. DNA encoding the open reading frame (ORF) for CFTR containing synonymous codon replacements was expressed using a heterologous vector integrated into the genome. The results indicate that the codon usage greatly affects the expression of CFTR. While the promoter strength driving expression of the ORFs was largely unchanged and the mRNA half-lives were unchanged, the steady-state levels of the mRNA varied by as much as 30-fold. Experiments support that this apparent inconsistency is attributed to nonsense mediated decay independent of exon junction complex. The ratio of CFTR/mRNA indicates that mRNA containing native codons was more efficient in expressing mature CFTR as compared to mRNA containing synonymous high-expression codons. However, when F508del CFTR was expressed after codon optimization, a greater percentage of the protein escaped endoplasmic-reticulum-associated degradation resulting in considerable levels of mature F508del CFTR on the plasma membrane, which showed channel activity. These results indicate that codon usage has an effect on mRNA levels and protein expression, for CFTR, and likely on chaperone-assisted folding pathway, for F508del CFTR.
Keywords: CFTR; CFTR F508del; nonsense mediated decay; protein folding; synonymous codon usage.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




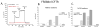
Similar articles
-
Increasing the Endoplasmic Reticulum Pool of the F508del Allele of the Cystic Fibrosis Transmembrane Conductance Regulator Leads to Greater Folding Correction by Small Molecule Therapeutics.PLoS One. 2016 Oct 12;11(10):e0163615. doi: 10.1371/journal.pone.0163615. eCollection 2016. PLoS One. 2016. PMID: 27732613 Free PMC article.
-
The silent codon change I507-ATC->ATT contributes to the severity of the ΔF508 CFTR channel dysfunction.FASEB J. 2013 Nov;27(11):4630-45. doi: 10.1096/fj.13-227330. Epub 2013 Aug 1. FASEB J. 2013. PMID: 23907436 Free PMC article.
-
F508del-CFTR increases intracellular Ca(2+) signaling that causes enhanced calcium-dependent Cl(-) conductance in cystic fibrosis.Biochim Biophys Acta. 2011 Nov;1812(11):1385-92. doi: 10.1016/j.bbadis.2011.08.008. Epub 2011 Aug 30. Biochim Biophys Acta. 2011. PMID: 21907281
-
Regulation of CFTR Biogenesis by the Proteostatic Network and Pharmacological Modulators.Int J Mol Sci. 2020 Jan 10;21(2):452. doi: 10.3390/ijms21020452. Int J Mol Sci. 2020. PMID: 31936842 Free PMC article. Review.
-
Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi.FEBS J. 2013 Sep;280(18):4396-406. doi: 10.1111/febs.12392. Epub 2013 Jul 5. FEBS J. 2013. PMID: 23773658 Review.
Cited by
-
Role for ribosome-associated complex and stress-seventy subfamily B (RAC-Ssb) in integral membrane protein translation.J Biol Chem. 2017 Dec 1;292(48):19610-19627. doi: 10.1074/jbc.M117.813857. Epub 2017 Oct 2. J Biol Chem. 2017. PMID: 28972146 Free PMC article.
-
Use of adenine base editing and homology-independent targeted integration strategies to correct the cystic fibrosis causing variant, W1282X.Hum Mol Genet. 2023 Nov 17;32(23):3237-3248. doi: 10.1093/hmg/ddad143. Hum Mol Genet. 2023. PMID: 37649273 Free PMC article.
-
Systematic deletion of symmetrical CFTR exons reveals new therapeutic targets for exon skipping antisense oligonucleotides.NAR Mol Med. 2024 Nov 6;1(4):ugae017. doi: 10.1093/narmme/ugae017. eCollection 2024 Oct. NAR Mol Med. 2024. PMID: 39582793 Free PMC article.
-
Mechanistic Approaches to Improve Correction of the Most Common Disease-Causing Mutation in Cystic Fibrosis.PLoS One. 2016 May 23;11(5):e0155882. doi: 10.1371/journal.pone.0155882. eCollection 2016. PLoS One. 2016. PMID: 27214033 Free PMC article.
-
Codon bias and the folding dynamics of the cystic fibrosis transmembrane conductance regulator.Cell Mol Biol Lett. 2016 Oct 19;21:23. doi: 10.1186/s11658-016-0025-x. eCollection 2016. Cell Mol Biol Lett. 2016. PMID: 28536625 Free PMC article. Review.
References
-
- CFTR_Gene_Family. http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&Term....
-
- Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–36. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

