Rapid and Profound Shifts in the Vaginal Microbiota Following Antibiotic Treatment for Bacterial Vaginosis
- PMID: 25676470
- PMCID: PMC4539900
- DOI: 10.1093/infdis/jiv079
Rapid and Profound Shifts in the Vaginal Microbiota Following Antibiotic Treatment for Bacterial Vaginosis
Abstract
Background: Bacterial vaginosis (BV) is a common polymicrobial disease associated with numerous negative reproductive health outcomes, including an increased risk of human immunodeficiency virus acquisition. BV is treatable with antibiotics, but relapse is common. A more detailed understanding of bacterial dynamics during antibiotic therapy for BV could identify conditions that favor establishment, maintenance, and eradication of BV-associated bacterial species, thereby improving treatment outcomes.
Methods: We used mathematical models to analyze daily quantitative measurements of 11 key bacterial species during metronidazole treatment for 15 cases of BV.
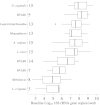
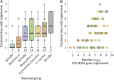
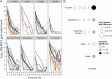
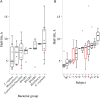
Results: We identified complete reorganization of vaginal bacterial composition within a day of initiating therapy. Although baseline bacterial levels predicted a longer time to clearance, all anaerobic species were eliminated rapidly within a median of 3 days. However, reemergence of BV-associated species was common following treatment cessation. Gardnerella vaginalis, a facultative anaerobe, was cleared more slowly than anaerobic BV-associated species, and levels of G. vaginalis often rebounded during treatment. We observed gradual Lactobacillus species growth, indicating that untargeted microbes fill the transient vacuum formed during treatment.
Conclusions: Under antibiotic pressure, the human microbiome can undergo rapid shifts on a scale of hours. When treatment is stopped, BV-associated bacteria quickly reemerge, suggesting a possible role for intermittent prophylactic treatment.
Keywords: Gardnerella vaginalis; Lactobacillus; bacterial vaginosis; mathematical modeling; metronidazole; qPCR; vaginal microbiota.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Haiser HJ, Turnbaugh PJ. Is it time for a metagenomic basis of therapeutics? Science 2012; 336:1253–5. - PubMed
-
- Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 34:864–9. - PubMed
-
- Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 1995; 333:1737–42. - PubMed
-
- Goldenberg RL, Thom E, Moawad AH, Johnson F, Roberts J, Cartis SN. The preterm prediction study: Fetal fibronectin, bacterial vaginosis, and peripartum infection. Obstet Gynecol 1996; 87:656–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

