Targeting Influenza A Virus RNA Promoter
- PMID: 25676805
- PMCID: PMC4531110
- DOI: 10.1111/cbdd.12534
Targeting Influenza A Virus RNA Promoter
Abstract
The emergence of drug-resistant strains of influenza virus makes exploring new classes of inhibitors that target universally conserved viral targets a highly important goal. The influenza A viral genome is made up of eight single-stranded RNA-negative segments. The RNA promoter, consisting of the conserved sequences at the 3' and 5' end of each RNA genomic segment, is universally conserved among influenza A virus strains and in all segments. Previously, we reported on the identification and NMR structure of DPQ (6,7-dimethoxy-2-(1-piperazinyl)-4-quinazolinamine) (compound 1) in complex with the RNA promoter. Here, we report on additional screening and SAR studies with compound 1, including ex vivo anti-influenza activity assays, resulted in improved cellular activity against influenza A virus in the micromolar range.
Keywords: chemical biology; drug design; drug discovery.
© 2015 John Wiley & Sons A/S.
Conflict of interest statement
The authors declare no financial/commercial conflicts of interest.
Figures

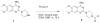

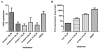
References
-
- Yoneda M, Okayama A, Kitahori Y. Oseltamivir-resistant seasonal A(H1N1) and A(H1N1)pdm09 influenza viruses from the 2007/2008 to 2012/2013 season in Nara Prefecture, Japan. Japanese journal of infectious diseases. 2014;67:385–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

