Alpha1-chimaerin, a Rac1 GTPase-activating protein, is expressed at reduced mRNA levels in the brain of Alzheimer's disease patients
- PMID: 25676811
- PMCID: PMC4382517
- DOI: 10.1016/j.neulet.2015.02.013
Alpha1-chimaerin, a Rac1 GTPase-activating protein, is expressed at reduced mRNA levels in the brain of Alzheimer's disease patients
Abstract
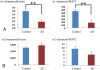
Alpha1-chimaerin is a GTPase-activating protein (GAP) for Rac1, a member of the Rho small GTPase family, whose action leads to the inactivation of Rac1. Rac1 activity is upregulated in Alzheimer's disease, but little is known about the role of α1-chimaerin. In this study, we investigated the expression and localization of α1-chimaerin mRNA in postmortem human brains from patients with Alzheimer's disease and control subjects. In situ hybridization studies demonstrated that α1-chimaerin was expressed by neurons in the neo-cortex of the temporal lobe and the hippocampus of both controls and Alzheimer's disease cases, with the signal intensity dramatically decreased in patients with Alzheimer's disease. Real-time PCR analysis confirmed a significant reduction of α1-chimaerin mRNA expression in the temporal cortex of Alzheimer's disease cases. In contrast, α2-chimaerin mRNA levels showed no significant difference between the groups. The present study showed reduced α1-chimaerin expression in the brain of Alzheimer's disease cases, suggesting a role in the upregulation of Rac1 activity during the disease process.
Keywords: Alzheimer’s disease; In situ hybridization; Rac1; Real-time PCR; α1-chimaerin.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures


Similar articles
-
alpha2-chimaerin, a Cdc42/Rac1 regulator, is selectively expressed in the rat embryonic nervous system and is involved in neuritogenesis in N1E-115 neuroblastoma cells.J Neurosci. 2001 Jul 15;21(14):5191-202. doi: 10.1523/JNEUROSCI.21-14-05191.2001. J Neurosci. 2001. PMID: 11438594 Free PMC article.
-
The diacylglycerol-binding protein alpha1-chimaerin regulates dendritic morphology.Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1924-9. doi: 10.1073/pnas.0510655103. Epub 2006 Jan 30. Proc Natl Acad Sci U S A. 2006. PMID: 16446429 Free PMC article.
-
RacGAP α2-chimaerin function in development adjusts cognitive ability in adulthood.Cell Rep. 2014 Sep 11;8(5):1257-64. doi: 10.1016/j.celrep.2014.07.047. Epub 2014 Aug 21. Cell Rep. 2014. PMID: 25159148
-
Rac GTPase-activating protein (Rac GAP) α1-Chimaerin undergoes proteasomal degradation and is stabilized by diacylglycerol signaling in neurons.J Biol Chem. 2011 Jan 7;286(1):199-207. doi: 10.1074/jbc.M110.166728. Epub 2010 Nov 5. J Biol Chem. 2011. PMID: 21056981 Free PMC article.
-
The Rac-GAP alpha2-chimaerin regulates hippocampal dendrite and spine morphogenesis.Mol Cell Neurosci. 2016 Sep;75:14-26. doi: 10.1016/j.mcn.2016.06.002. Epub 2016 Jun 11. Mol Cell Neurosci. 2016. PMID: 27297944 Free PMC article.
Cited by
-
Rho GTPases in Intellectual Disability: From Genetics to Therapeutic Opportunities.Int J Mol Sci. 2018 Jun 20;19(6):1821. doi: 10.3390/ijms19061821. Int J Mol Sci. 2018. PMID: 29925821 Free PMC article. Review.
-
Molecular differences in brain regional vulnerability to aging between males and females.Front Aging Neurosci. 2023 May 22;15:1153251. doi: 10.3389/fnagi.2023.1153251. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37284017 Free PMC article.
-
Regulators of Rho GTPases in the Nervous System: Molecular Implication in Axon Guidance and Neurological Disorders.Int J Mol Sci. 2019 Mar 25;20(6):1497. doi: 10.3390/ijms20061497. Int J Mol Sci. 2019. PMID: 30934641 Free PMC article. Review.
-
Roles of Rac1-Dependent Intrinsic Forgetting in Memory-Related Brain Disorders: Demon or Angel.Int J Mol Sci. 2023 Jun 27;24(13):10736. doi: 10.3390/ijms241310736. Int J Mol Sci. 2023. PMID: 37445914 Free PMC article. Review.
-
Dysregulation of Exosome Cargo by Mutant Tau Expressed in Human-induced Pluripotent Stem Cell (iPSC) Neurons Revealed by Proteomics Analyses.Mol Cell Proteomics. 2020 Jun;19(6):1017-1034. doi: 10.1074/mcp.RA120.002079. Epub 2020 Apr 15. Mol Cell Proteomics. 2020. PMID: 32295833 Free PMC article.
References
-
- Hall C, Monfries C, Smith P, Lim HH, Kozma R, Ahmed S, Vanniasingham V, Leung T, Lim L. Novel human brain cDNA encoding a 34,000 Mr protein n-chimaerin, related to both the regulatory domain of protein kinase C and BCR, the product of the breakpoint cluster region gene. J Mol Biol. 1990;211:11–16. - PubMed
-
- Luo L, Hensch TK, Ackeman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkine cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

