Clarifying the morphology of the ostium primum defect
- PMID: 25676858
- PMCID: PMC4337664
- DOI: 10.1111/joa.12272
Clarifying the morphology of the ostium primum defect
Abstract
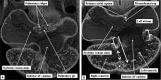
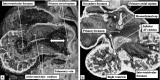
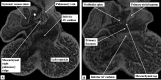
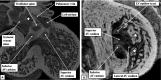
The 'ostium primum' defect is still frequently considered to be the consequence of deficient atrial septation, although the key feature is a common atrioventricular junction. The bridging leaflets of the common atrioventricular valve, which are joined to each other, are depressed distal to the atrioventricular junction, and fused to the crest of the muscular ventricular septum, which is bowed in the concave direction towards the ventricular apex. As a result, shunting across the defect occurs between the atrial chambers. These observations suggest that the basic deficiency in the 'ostium primum' defect is best understood as a product of defective atrioventricular septation, rather than an atrial septal defect. We have now encountered four examples of 'ostium primum' defects in mouse embryos that support this view. These were identified from a large number of mouse embryo hearts collected from a normal, outbred mouse colony and analysed by episcopic microscopy as part of an ongoing study of normal mouse cardiac development. The abnormal hearts were identified from embryos collected at embryonic days 15.5, 16.5 and 18.5 (two cases). We have analysed the features of the abnormal hearts, and compared the findings with those obtained in the large number of normally developed embryos. Our data show that the key feature of normal atrioventricular septation is the ventral growth through the right pulmonary ridge of a protrusion from the dorsal pharyngeal mesenchyme, confirming previous findings. This protrusion, known as the vestibular spine, or the dorsal mesenchymal protrusion, reinforces the closure of the primary atrial foramen, and muscularises along with the mesenchymal cap of the primary atrial septum to form the ventro-caudal buttress of the oval foramen, identified by some as the 'canal septum'. Detailed analysis of the four abnormal hearts suggests that in each case there has been failure of growth of the vestibular spine, with the result that the common atrioventricular junction found earlier during normal development now persists during cardiac development. Failure of separation of the common junction also accounts for the trifoliate arrangement of the left atrioventricular valve in the abnormal hearts. Analysis of the episcopic datasets also permits recognition of the location of the atrioventricular conduction axis. Comparison of the location of this tract in the normal and abnormal hearts shows that there is no separate formation of a ventricular component of the 'canal septum' as part of normal development. We conclude that it is abnormal formation of the primary atrial septum that is the cause of so-called 'secundum' atrial septal defects, whereas it is the failure to produce a second contribution to atrial septation (via growth of the vestibular spine) that results in the 'ostium primum' defect.
Keywords: atrioventricular septal defect; endocardial cushions; heart; primary atrial septum; vestibular spine.
© 2015 Anatomical Society.
Figures
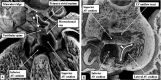
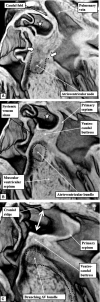



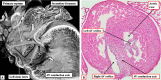
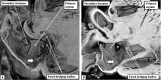
Similar articles
-
Deficiency of the vestibular spine in atrioventricular septal defects in human fetuses with down syndrome.Am J Cardiol. 2003 Jan 15;91(2):180-4. doi: 10.1016/s0002-9149(02)03106-5. Am J Cardiol. 2003. PMID: 12521631
-
Morphology and morphogenesis of atrioventricular septal defect with common atrioventricular junction.World J Pediatr Congenit Heart Surg. 2010 Apr;1(1):59-67. doi: 10.1177/2150135109360813. World J Pediatr Congenit Heart Surg. 2010. PMID: 23804724
-
Morphologic features of atrioventricular septal defect with only ventricular component: further observations pertinent to surgical repair.J Thorac Cardiovasc Surg. 2009 Jan;137(1):132-8, 138.e1-2. doi: 10.1016/j.jtcvs.2008.03.032. Epub 2008 May 19. J Thorac Cardiovasc Surg. 2009. PMID: 19154915
-
Molecular Pathways and Animal Models of Atrial Septal Defect.Adv Exp Med Biol. 2024;1441:481-493. doi: 10.1007/978-3-031-44087-8_25. Adv Exp Med Biol. 2024. PMID: 38884727 Review.
-
The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion.Differentiation. 2012 Jul;84(1):117-30. doi: 10.1016/j.diff.2012.05.006. Epub 2012 Jun 17. Differentiation. 2012. PMID: 22709652 Free PMC article. Review.
Cited by
-
Making the most of episcopic datasets from developing mice.J Anat. 2022 Mar;240(3):589-590. doi: 10.1111/joa.13573. Epub 2021 Oct 27. J Anat. 2022. PMID: 34708411 Free PMC article. No abstract available.
-
The Mesenchymal Cap of the Atrial Septum and Atrial and Atrioventricular Septation.J Cardiovasc Dev Dis. 2020 Nov 4;7(4):50. doi: 10.3390/jcdd7040050. J Cardiovasc Dev Dis. 2020. PMID: 33158164 Free PMC article. Review.
-
Visualising the Cardiovascular System of Embryos of Biomedical Model Organisms with High Resolution Episcopic Microscopy (HREM).J Cardiovasc Dev Dis. 2018 Dec 15;5(4):58. doi: 10.3390/jcdd5040058. J Cardiovasc Dev Dis. 2018. PMID: 30558275 Free PMC article. Review.
-
The Fate of the Outflow Tract Septal Complex in Relation to the Classification of Ventricular Septal Defects.J Cardiovasc Dev Dis. 2019 Feb 21;6(1):9. doi: 10.3390/jcdd6010009. J Cardiovasc Dev Dis. 2019. PMID: 30795606 Free PMC article. Review.
-
Cardiac multi-scale investigation of the right and left ventricle ex vivo: a review.Cardiovasc Diagn Ther. 2020 Oct;10(5):1701-1717. doi: 10.21037/cdt-20-269. Cardiovasc Diagn Ther. 2020. PMID: 33224784 Free PMC article. Review.
References
-
- Anderson RH, Wessels A, Vettukattil J. Morphology and morphogenesis of atrioventricular septal defect with common atrioventricular junction. World J Pediatr Congenit Heart Surg. 2010;1:59–67. - PubMed
-
- Anderson RH, Spicer DE, Brown NA, et al. The development of septation in the four-chambered heart. Anat Rec (Hoboken) 2014;297:1414–1429. - PubMed
-
- Aschoff L. Referat uber die Herzstorungen in ihren Beziehungen zu den Spezifischen Muskelsystem des Herzens. Verh Dtsch Pathol Ges. 1910;14:3–35.
-
- Becker AE, Anderson RH. Atrioventricular septal defects. What's in a name? J Thorac Cardiovasc Surg. 1982;83:461–469. - PubMed
-
- Blom NA, Ottenkamp J, Wenick ACG, et al. Deficiency of the vestibular spine in atrioventricular septal defects in human foetuses with Down syndrome. Am J Cardiol. 2003;91:180–184. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

