Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing
- PMID: 25676876
- PMCID: PMC4517185
- DOI: 10.1002/jor.22855
Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing
Abstract
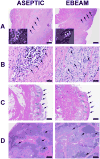
Extracellular matrix (ECM) scaffolds have been used to enhance anterior cruciate ligament (ACL) repair in large animal models. To translate this technology to clinical care, identifying a method which effectively sterilizes the material without significantly impairing in vivo function is desirable. Sixteen Yorkshire pigs underwent ACL transection and were randomly assigned to bridge-enhanced ACL repair-primary suture repair of the ACL with addition of autologous blood soaked ECM scaffold--with either (i) an aseptically processed ECM scaffold, or (ii) an electron beam irradiated ECM scaffold. Primary outcome measures included sterility of the scaffold and biomechanical properties of the scaffold itself and the repaired ligament at 8 weeks after surgery. Scaffolds treated with 15 kGy electron beam irradiation had no bacterial or fungal growth noted, while aseptically processed scaffolds had bacterial growth in all tested samples. The mean biomechanical properties of the scaffold and healing ligament were lower in the electron beam group; however, differences were not statistically significant. Electron beam irradiation was able to effectively sterilize the scaffolds. In addition, this technique had only a minimal impact on the in vivo function of the scaffolds when used for ligament healing in the porcine model.
Keywords: anterior cruciate ligament; bridge-enhanced ACL repair; collagen scaffold; electron beam irradiation.
© 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


References
-
-
11139:2006. IT. 2006. Sterilization of health care products - vocabulary.
-
-
- Favero M. In: Sterility assurance: concepts for patient safety, in disinfection, sterilization and antisepsis: principles and practices in healthcare facilities, chapter 12. WA R, editor. Washington: Association for Professionals in Infection Control and Epidemiology. DC.; 2001. pp. 110–119.
-
-
ANSI/AAMI_ST67:2003. 203. Sterilization of health care products - requirements for products labeled “STERILE”.
-
-
- Hemmerich KJ, et al. Sterilization methods stand the test of time. Medical Device and Diagnostic Industry. 2004;26
-
- Daly KA, Liu S, Agrawal V, et al. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials. 2012;33:91–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

