A randomized trial of levodopa as treatment for residual amblyopia in older children
- PMID: 25676904
- PMCID: PMC4414716
- DOI: 10.1016/j.ophtha.2015.01.002
A randomized trial of levodopa as treatment for residual amblyopia in older children
Abstract
Objective: To assess the efficacy and short-term safety of levodopa as adjunctive treatment to patching for amblyopia.
Design: Randomized, placebo-controlled trial.
Participants: One hundred thirty-nine children 7 to 12 years of age with residual amblyopia resulting from strabismus, anisometropia, or both combined (visual acuity [VA], 20/50-20/400) after patching.
Methods: Sixteen weeks of oral levodopa or placebo administered 3 times daily while patching the fellow eye 2 hours daily.
Main outcome measures: Mean change in best-corrected amblyopic-eye VA at 18 weeks.
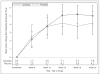
Results: At 18 weeks, amblyopic-eye VA improved from randomization by an average of 5.2 letters in the levodopa group and by 3.8 letters in the placebo group (difference adjusted for baseline VA, +1.4 letters; 1-sided P=0.06; 2-sided 95% confidence interval, -0.4 to 3.3 letters). No serious adverse effects from levodopa were reported during treatment.
Conclusions: For children 7 to 12 years of age with residual amblyopia after patching therapy, oral levodopa while continuing to patch 2 hours daily does not produce a clinically or statistically meaningful improvement in VA compared with placebo and patching.
Copyright © 2015 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
What is next in amblyopia treatment?Ophthalmology. 2015 May;122(5):871-3. doi: 10.1016/j.ophtha.2015.02.015. Ophthalmology. 2015. PMID: 25919778 No abstract available.
References
-
- Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005;123(4):437–47. - PubMed
-
- Pediatric Eye Disease Investigator Group. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2005;123(2):149–57. - PubMed
-
- Leguire LE, Walson PD, Rogers GL, et al. Levodopa/carbidopa treatment for amblyopia in older children. J Pediatr Ophthalmol Strabismus. 1995;32(3):143–51. - PubMed
-
- Procianoy E, Fuchs FD, Procianoy L, Procianoy F. The effect of increasing doses of levodopa on children with strabismic amblyopia. J AAPOS. 1999;3(6):337–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical