Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review
- PMID: 25677107
- PMCID: PMC4594723
- DOI: 10.1111/bcp.12600
Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review
Abstract
In order to reduce the numbers of medication errors (MEs) that cause adverse reactions (ARs) many authors have tried to identify patient-related risk factors. However, the evidence remains controversial. The aim was to review systematically the evidence on the relationship between patient-related risk factors and the risk of serious ARs. A systematic search in Pubmed, Embase, Cochrane Systematic Reviews, Psychinfo and SweMed+ was performed. Included full text articles were hand searched for further references. Peer reviewed papers including adults from primary and secondary healthcare were included if they clearly defined seriousness of the ARs and described correlations to risk factors by statistical analysis. A total of 28 studies were identified including 85,212 patients with 3385 serious ARs, resulting in an overall frequency of serious ARs in 4% of patients. Age, gender and number of drugs were by far the most frequently investigated risk factors. The total number of drugs was the most consistent correlated risk factor found in both univariate and multivariate analyses. The number of drugs is the most frequently documented independent patient-related risk factor for serious ARs in both the general adult population as well as in the elderly. The existing evidence is however conflicting due to heterogeneity of populations and study methods. The knowledge of patient-related risk factors for experiencing ARs could be used for electronic risk stratification of patients and thereby allocation of healthcare resources to high risk patients.
Keywords: drug-related side effects and adverse reactions; medication errors; risk factors.
© 2015 The British Pharmacological Society.
Figures
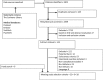

 Studies including only the elderly and ARs as cause of hospitalization.
Studies including only the elderly and ARs as cause of hospitalization.  Studies including all adults and ARs as cause of hospitalization.
Studies including all adults and ARs as cause of hospitalization.  Studies including ARs during hospitalization (all adults).
Studies including ARs during hospitalization (all adults).  AR, Adverse reaction.
AR, Adverse reaction.References
-
- Guideline on good pharmacovigilance practices - Module VI. Management and reporting of adverse reactions to medicinal products. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general... (last accessed 18 December 2013)
-
- 2013. E2AClinical safety data management: Definitions and standards for expedited reporting. Available at www.ich.org (last accessed 4 July 2013)
-
- Pardo Cabello AJ, Gonzalez Contreras LG, Manzano Gamero MV, Gomez Jimenez FJ, Puche Canas E. Prevalence of fatal adverse drug reactions in hospitalized patients. Int J Clin Pharmacol Ther. 2009;47:596–602. - PubMed
-
- Buajordet I, Ebbesen J, Erikssen J, Brors O, Hilberg T. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250:327–41. - PubMed
-
- Ebbesen J, Buajordet I, Erikssen J, Brors O, Hilberg T, Svaar H, Sandvik L. Drug-related deaths in a Department of Internal Medicine. Arch Intern Med. 2001;161:2317–23. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

