Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial
- PMID: 25677354
- PMCID: PMC4451432
- DOI: 10.1176/appi.ajp.2014.14070889
Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial
Abstract
Objective: The authors evaluated the potential of methylphenidate to improve antidepressant response to citalopram, as assessed by clinical and cognitive outcomes, in elderly depressed patients.
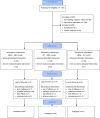
Method: The authors conducted a 16-week randomized double-blind placebo-controlled trial for geriatric depression in 143 older outpatients diagnosed with major depression comparing treatment response in three treatment groups: methylphenidate plus placebo (N=48), citalopram plus placebo (N=48), and citalopram plus methylphenidate (N=47). The primary outcome measure was change in depression severity. Remission was defined as a score of 6 or less on the Hamilton Depression Rating Scale. Secondary outcomes included measures of anxiety, apathy, quality of life, and cognition.
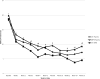
Results: Daily doses ranged from 20 mg to 60 mg for citalopram (mean=32 mg) and from 5 mg to 40 mg for methylphenidate (mean=16 mg). All groups showed significant improvement in depression severity and in cognitive performance. However, the improvement in depression severity and the Clinical Global Impressions improvement score was more prominent in the citalopram plus methylphenidate group compared with the other two groups. Additionally, the rate of improvement in the citalopram plus methylphenidate group was significantly higher than that in the citalopram plus placebo group in the first 4 weeks of the trial. The groups did not differ in cognitive improvement or number of side effects.
Conclusions: Combined treatment with citalopram and methylphenidate demonstrated an enhanced clinical response profile in mood and well-being, as well as a higher rate of remission, compared with either drug alone. All treatments led to an improvement in cognitive functioning, although augmentation with methylphenidate did not offer additional benefits.
Conflict of interest statement
Figures
Comment in
-
The role of stimulants in late-life depression.Am J Psychiatry. 2015 Jun;172(6):505-7. doi: 10.1176/appi.ajp.2015.15030356. Am J Psychiatry. 2015. PMID: 26029800 No abstract available.
-
Cardiac Effects of Methylphenidate.Am J Psychiatry. 2015 Oct;172(10):1023. doi: 10.1176/appi.ajp.2015.15060795. Am J Psychiatry. 2015. PMID: 26423484 No abstract available.
-
Response to Roose and Rutherford.Am J Psychiatry. 2015 Oct;172(10):1023-4. doi: 10.1176/appi.ajp.2015.15060795r. Am J Psychiatry. 2015. PMID: 26423485 No abstract available.
References
-
- Alexopoulos GS, Young RC, Abrams RC, Meyers B, Shamoian CA. Chronicity and relapse in geriatric depression. Biol Psychiatry. 1989;26(6):551–64. - PubMed
-
- Reynolds CF, 3rd, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130–8. - PubMed
-
- Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008;16(7):558–67. Epub 2008/07/02. - PubMed
-
- Nelson JC, Delucchi KL, Schneider LS. Moderators of outcome in late-life depression: a patient-level meta-analysis. Am J Psychiatry. 2013;170(6):651–9. Epub 2013/04/20. - PubMed

