Corticosteroids in IgA Nephropathy: A Retrospective Analysis from the VALIGA Study
- PMID: 25677392
- PMCID: PMC4552116
- DOI: 10.1681/ASN.2014070697
Corticosteroids in IgA Nephropathy: A Retrospective Analysis from the VALIGA Study
Abstract
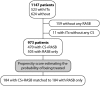
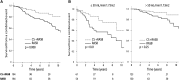
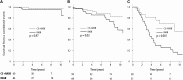
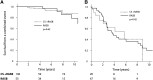
Current guidelines suggest treatment with corticosteroids (CS) in IgA nephropathy (IgAN) when proteinuria is persistently ≥1 g/d despite 3-6 months of supportive care and when eGFR is >50 ml/min per 1.73 m(2). Whether the benefits of this treatment extend to patients with an eGFR≤50 ml/min per 1.73 m(2), other levels of proteinuria, or different renal pathologic lesions remains unknown. We retrospectively studied 1147 patients with IgAN from the European Validation Study of the Oxford Classification of IgAN (VALIGA) cohort classified according to the Oxford-MEST classification and medication used, with details of duration but not dosing. Overall, 46% of patients received immunosuppression, of which 98% received CS. Treated individuals presented with greater clinical and pathologic risk factors of progression. They also received more antihypertensive medication, and a greater proportion received renin angiotensin system blockade (RASB) compared with individuals without immunosuppressive therapy. Immunosuppression was associated with a significant reduction in proteinuria, a slower rate of renal function decline, and greater renal survival. Using a propensity score, we matched 184 subjects who received CS and RASB to 184 patients with a similar risk profile of progression who received only RASB. Within this group, CS reduced proteinuria and the rate of renal function decline and increased renal survival. These benefits extended to those with an eGFR≤50 ml/min per 1.73 m(2), and the benefits increased proportionally with the level of proteinuria. Thus, CS reduced the risk of progression regardless of initial eGFR and in direct proportion to the extent of proteinuria in this cohort.
Keywords: IgA nephropathy; immunosuppression; pathology; progression of chronic renal failure; proteinuria; risk factors.
Copyright © 2015 by the American Society of Nephrology.
Figures
Comment in
-
IgA Nephritis with Declining Renal Function: Treatment with Corticosteroids May Be Worthwhile.J Am Soc Nephrol. 2015 Sep;26(9):2071-3. doi: 10.1681/ASN.2015010030. Epub 2015 Feb 12. J Am Soc Nephrol. 2015. PMID: 25677390 Free PMC article. No abstract available.
-
Glomerular disease: Efficacy of corticosteroids in high-risk IgA nephropathy.Nat Rev Nephrol. 2015 Jun;11(6):319-20. doi: 10.1038/nrneph.2015.47. Epub 2015 Apr 7. Nat Rev Nephrol. 2015. PMID: 25848878 No abstract available.
References
-
- Radford MG, Jr, Donadio JV, Jr, Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 - PubMed
-
- Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 28: 4–9, 2008 - PubMed
-
- Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—Application to the individual patient. Kidney Int 82: 840–856, 2012 - PubMed
-
- Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

