Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study
- PMID: 25677679
- PMCID: PMC4392202
- DOI: 10.1136/thoraxjnl-2014-206345
Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study
Abstract
Background: QVA149 is a once-daily (o.d.) inhaled dual bronchodilator containing a fixed-dose combination of the long-acting β2-agonist indacaterol and the long-acting muscarinic antagonist glycopyrronium for the treatment of COPD. The QUANTIFY study compared QVA149 with a free-dose bronchodilator combination of tiotropium plus formoterol (TIO+FOR) in improving health-related quality of life (HRQoL) of patients with COPD.
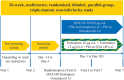
Methods: This multicentre, blinded, triple-dummy, parallel-group, non-inferiority study randomised patients aged ≥40 years with moderate-to-severe COPD (post-bronchodilator forced expiratory volume in 1 s (FEV1) ≥30% to <80% predicted) to QVA149 110/50 µg o.d. or TIO 18 µg o.d.+ FOR 12 µg twice daily (1:1) for 26 weeks. The primary endpoint was to demonstrate non-inferiority in HRQoL assessed using St George's Respiratory Questionnaire-COPD (SGRQ-C). The prespecified non-inferiority margin was 4 units. Secondary endpoints included Transition Dyspnoea Index (TDI) score, pre-dose FEV1, forced vital capacity (FVC) and safety.
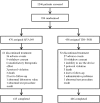
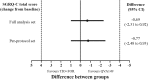
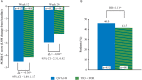
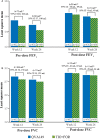
Results: Of the 934 patients randomised (QVA149=476 and TIO+FOR=458), 87.9% completed the study. At week 26, non-inferiority was met for SGRQ-C (QVA149 vs TIO+FOR; difference: -0.69 units; 95% CI -2.31 to 0.92; p=0.399). A significantly higher percentage of patients achieved a clinically relevant ≥1 point improvement in TDI total score with QVA149 (49.6%) versus TIO+FOR (42.4%; p=0.033). QVA149 significantly increased pre-dose FEV1 (+68 mL, 95% CI 37 mL to 100 mL; p<0.001) and FVC (+74 mL, 95% CI 24 mL to 125 mL; p=0.004) compared with TIO+FOR at week 26. The incidence of adverse events was comparable between both treatments (QVA149=43.7% and TIO+FOR=42.6%).
Conclusions: QVA149 is non-inferior to TIO+FOR in improving HRQoL, with clinically meaningful and significant improvements in breathlessness and lung function in patients with COPD.
Trial registration number: NCT01120717.
Keywords: COPD Pharmacology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London, 2010. https://www.nice.org.uk/guidance/qs10
-
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2014. http://www.goldcopd.com/
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
