How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila
- PMID: 25677943
- PMCID: PMC4326961
- DOI: 10.1038/srep08454
How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila
Abstract
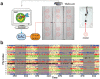
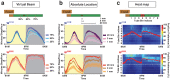
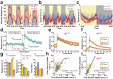
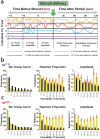
The fruitfly, Drosophila melanogaster, has become a critical model system for investigating sleep functions. Most studies use duration of inactivity to measure sleep. However, a defining criterion for sleep is decreased behavioral responsiveness to stimuli. Here we introduce the Drosophila ARousal Tracking system (DART), an integrated platform for efficiently tracking and probing arousal levels in animals. This video-based platform delivers positional and locomotion data, behavioral responsiveness to stimuli, sleep intensity measures, and homeostatic regulation effects - all in one combined system. We show how insight into dynamically changing arousal thresholds is crucial for any sleep study in flies. We first find that arousal probing uncovers different sleep intensity profiles among related genetic background strains previously assumed to have equivalent sleep patterns. We then show how sleep duration and sleep intensity can be uncoupled, with distinct manipulations of dopamine function producing opposite effects on sleep duration but similar sleep intensity defects. We conclude by providing a multi-dimensional assessment of combined arousal and locomotion metrics in the mutant and background strains. Our approach opens the door for deeper insights into mechanisms of sleep regulation and provides a new method for investigating the role of different genetic manipulations in controlling sleep and arousal.
Figures

Similar articles
-
Dopamine is a regulator of arousal in the fruit fly.J Neurosci. 2005 Aug 10;25(32):7377-84. doi: 10.1523/JNEUROSCI.2048-05.2005. J Neurosci. 2005. PMID: 16093388 Free PMC article.
-
Sleep homeostasis in Drosophila melanogaster.Sleep. 2004 Jun 15;27(4):628-39. doi: 10.1093/sleep/27.4.628. Sleep. 2004. PMID: 15282997
-
Deep conservation of genes required for both Drosphila melanogaster and Caenorhabditis elegans sleep includes a role for dopaminergic signaling.Sleep. 2014 Sep 1;37(9):1439-51. doi: 10.5665/sleep.3990. Sleep. 2014. PMID: 25142568 Free PMC article.
-
[Regulation of sleep and arousal in Drosophila].Seikagaku. 2007 Jan;79(1):39-42. Seikagaku. 2007. PMID: 17319512 Review. Japanese. No abstract available.
-
Dopamine in Drosophila: setting arousal thresholds in a miniature brain.Proc Biol Sci. 2011 Mar 22;278(1707):906-13. doi: 10.1098/rspb.2010.2564. Epub 2011 Jan 5. Proc Biol Sci. 2011. PMID: 21208962 Free PMC article. Review.
Cited by
-
Sleep benefits different stages of memory in Drosophila.Front Physiol. 2023 Jan 19;14:1087025. doi: 10.3389/fphys.2023.1087025. eCollection 2023. Front Physiol. 2023. PMID: 36744027 Free PMC article. Review.
-
Acute control of the sleep switch in Drosophila reveals a role for gap junctions in regulating behavioral responsiveness.Elife. 2018 Aug 15;7:e37105. doi: 10.7554/eLife.37105. Elife. 2018. PMID: 30109983 Free PMC article.
-
Identification of octopaminergic neurons that modulate sleep suppression by male sex drive.Elife. 2017 May 16;6:e23130. doi: 10.7554/eLife.23130. Elife. 2017. PMID: 28510528 Free PMC article.
-
Rest, Repair, Repeat: The Complex Relationship of Autophagy and Sleep.J Mol Biol. 2025 Sep 15;437(18):169227. doi: 10.1016/j.jmb.2025.169227. Epub 2025 May 21. J Mol Biol. 2025. PMID: 40409707 Review.
-
Variant-to-gene mapping followed by cross-species genetic screening identifies GPI-anchor biosynthesis as a regulator of sleep.Sci Adv. 2023 Jan 6;9(1):eabq0844. doi: 10.1126/sciadv.abq0844. Epub 2023 Jan 6. Sci Adv. 2023. PMID: 36608130 Free PMC article.
References
-
- Shaw P. J., Cirelli C., Greenspan R. J. & Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000). - PubMed
-
- Hendricks J. C. et al. Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000). - PubMed
-
- Tononi G. & Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62, 143–150 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

