The subventricular zone in the immature piglet brain: anatomy and exodus of neuroblasts into white matter after traumatic brain injury
- PMID: 25678047
- PMCID: PMC4406780
- DOI: 10.1159/000369091
The subventricular zone in the immature piglet brain: anatomy and exodus of neuroblasts into white matter after traumatic brain injury
Abstract
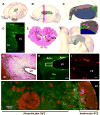
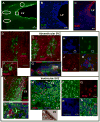
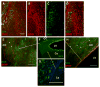
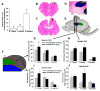
Stimulation of postnatal neurogenesis in the subventricular zone (SVZ) and robust migration of neuroblasts to the lesion site in response to traumatic brain injury (TBI) is well established in rodent species; however, it is not yet known whether postnatal neurogenesis plays a role in repair after TBI in gyrencephalic species. Here we describe the anatomy of the SVZ in the piglet for the first time and initiate an investigation into the effect of TBI on the SVZ architecture and the number of neuroblasts in the white matter. Among all ages of immaturity examined the SVZ contained a dense mesh network of neurogenic precursor cells (doublecortin+) positioned directly adjacent to the ependymal cells (ventricular SVZ, Vsvz) and neuroblasts organized into chains that were distinct from the Vsvz (abventricular SVZ, Asvz). Though the architecture of the SVZ was similar among ages, the areas of Vsvz and Asvz neuroblast chains declined with age. At postnatal day (PND) 14 the white matter tracts have a tremendous number of individual neuroblasts. In our scaled cortical impact model, lesion size increased with age. Similarly, the response of the SVZ to injury was also age dependent. The younger age groups that sustained the proportionately smallest lesions had the largest SVZ areas, which further increased in response to injury. In piglets that were injured at 4 months of age and had the largest lesions, the SVZ did not increase in response to injury. Similar to humans, swine have abundant gyri and gyral white matter, providing a unique platform to study neuroblasts potentially migrating from the SVZ to the lesioned cortex along these white matter tracts. In piglets injured at PND 7, TBI did not increase the total number of neuroblasts in the white matter compared to uninjured piglets, but redistribution occurred with a greater number of neuroblasts in the white matter of the hemisphere ipsilateral to the injury compared to the contralateral hemisphere. At 7 days after injury, less than 1% of neuroblasts in the white matter were born in the 2 days following injury. These data show that the SVZ in the piglet shares many anatomical similarities with the SVZ in the human infant, and that TBI had only modest effects on the SVZ and the number of neuroblasts in the white matter. Piglets at an equivalent developmental stage to human infants were equipped with the largest SVZ and a tremendous number of neuroblasts in the white matter, which may be sufficient in lesion repair without the dramatic stimulation of neurogenic machinery. It has yet to be determined whether neurogenesis and migrating neuroblasts play a role in repair after TBI and/or whether an alteration of normal migration during active postnatal population of brain regions is beneficial in species with gyrencephalic brains.
© 2015 S. Karger AG, Basel.
Figures


References
-
- Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007 Dec 13;1(6):612–27. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969 Dec;137(4):433–57. - PubMed
-
- Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol. 2006 Oct 1;498(4):491–507. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

