Defects of mutant DNMT1 are linked to a spectrum of neurological disorders
- PMID: 25678562
- PMCID: PMC5014076
- DOI: 10.1093/brain/awv010
Defects of mutant DNMT1 are linked to a spectrum of neurological disorders
Abstract
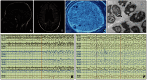
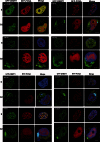
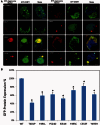
We report a broader than previously appreciated clinical spectrum for hereditary sensory and autonomic neuropathy type 1E (HSAN1E) and a potential pathogenic mechanism for DNA methyltransferase (DNMT1) mutations. The clinical presentations and genetic characteristics of nine newly identified HSAN1E kinships (45 affected subjects) were investigated. Five novel mutations of DNMT1 were discovered; p.C353F, p.T481P, p.P491L, p.Y524D and p.I531N, all within the target-sequence domain, and two mutations (p.T481P, p.P491L) arising de novo. Recently, HSAN1E has been suggested as an allelic disorder of autosomal dominant cerebellar ataxia, deafness and narcolepsy. Our results indicate that all the mutations causal for HSAN1E are located in the middle part or N-terminus end of the TS domain, whereas all the mutations causal for autosomal dominant cerebellar ataxia, deafness and narcolepsy are located in the C-terminus end of the TS domain. The impact of the seven causal mutations in this cohort was studied by cellular localization experiments. The binding efficiency of the mutant DNMT proteins at the replication foci and heterochromatin were evaluated. Phenotypic characterizations included electromyography, brain magnetic resonance and nuclear imaging, electroencephalography, sural nerve biopsies, sleep evaluation and neuropsychometric testing. The average survival of HSAN1E was 53.6 years. [standard deviation = 7.7, range 43-75 years], and mean onset age was 37.7 years. (standard deviation = 8.6, range 18-51 years). Expanded phenotypes include myoclonic seizures, auditory or visual hallucinations, and renal failure. Hypersomnia, rapid eye movement sleep disorder and/or narcolepsy were identified in 11 subjects. Global brain atrophy was found in 12 of 14 who had brain MRI. EEGs showed low frequency (delta waves) frontal-predominant abnormality in five of six patients. Marked variability in cognitive deficits was observed, but the majority of patients (89%) developed significant cognitive deficit by the age of 45 years. Cognitive function decline often started with personality changes and psychiatric manifestations. A triad of hearing loss, sensory neuropathy and cognitive decline remains as the stereotypic presentation of HSAN1E. Moreover, we show that mutant DNMT1 proteins translocate to the cytoplasm and are prone to form aggresomes while losing their binding ability to heterochromatin during the G2 cell cycle. Our results suggest mutations in DNMT1 result in imbalanced protein homeostasis through aggresome-induced autophagy. This mechanism may explain why mutations in the sole DNA maintenance methyltransferase lead to selective central and peripheral neurodegeneration.
Keywords: REM sleep behaviour disorder; narcolepsy; neurodegeneration; protein aggregation; sensory neuropathy.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–6. - PubMed
-
- Drmanac R, Sparks AB, Callow MJ, Halpern AL, Burns NL, Kermani BG, et al. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. 2010;327:78–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10NS077305/NS/NINDS NIH HHS/United States
- G1001253/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- T32 AG023481/AG/NIA NIH HHS/United States
- R01 DK064814/DK/NIDDK NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- G0802760/MRC_/Medical Research Council/United Kingdom
- G0601943/MRC_/Medical Research Council/United Kingdom
- DP3 DK104394/DK/NIDDK NIH HHS/United States
- K08 NS065007/NS/NINDS NIH HHS/United States
- U54 NS065712/NS/NINDS NIH HHS/United States
- R01 CA132878/CA/NCI NIH HHS/United States
- G108/638/MRC_/Medical Research Council/United Kingdom
- MR/J004758/1/MRC_/Medical Research Council/United Kingdom
- U54NS065712/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- DP3DK104394/DK/NIDDK NIH HHS/United States
- NS065007/NS/NINDS NIH HHS/United States
- R01DK064814/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

