Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion
- PMID: 25678696
- PMCID: PMC4398886
- DOI: 10.1152/japplphysiol.00313.2014
Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion
Abstract
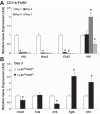
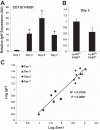
The presence of macrophages (MPs) is essential for skeletal muscle to properly regenerate following injury. The aim of this study was the evaluation of MP profiles and their importance in skeletal muscle recovering from tourniquet-induced ischemia-reperfusion (I/R). Using flow cytometry, we identified two distinct CD11b(+) MP populations that differ in expression of the surface markers Ly-6C and F4/80. These populations are prominent at 3 and 5 days of reperfusion and molecularly correspond to inflammatory and anti-inflammatory MP phenotypes. Sorted MP populations demonstrated high levels of IGF-I expression, and whole muscle post-I/R IGF-I expression strongly correlates with F4/80 expression. This suggests MPs largely influence postinjury IGF-I upregulation. We additionally demonstrate that direct intramuscular injection of FACS-isolated CD11b(+)Ly-6C(lo)F4/80(hi) MPs improves the functional and histological recovery of I/R-affected muscle. Taken together, these data further support the substantial influence of the innate immune system on muscle regeneration and suggest MP-focused therapeutic approaches may greatly facilitate skeletal muscle recovery from substantial injury.
Keywords: flow cytometry; macrophage; myogenesis; regenerative medicine; reperfusion.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, Aamiri A, Di Santo JP, Chazaud B, Butler-Browne G, Savino W, Mouly V, Riederer I. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther 20: 2168–2179, 2012. - PMC - PubMed
-
- Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 10: 620–630, 2002. - PubMed
-
- Bryer SC, Fantuzzi G, Van Rooijen N, Koh TJ. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J Immunol 180: 1179–1188, 2008. - PubMed
-
- Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci 23: 189–194, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

