Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence
- PMID: 25678956
- PMCID: PMC4326185
- DOI: 10.1186/2045-8118-11-26
Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence
Abstract
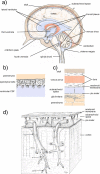
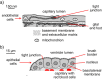
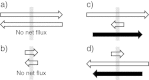
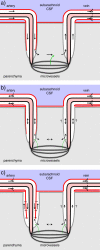
Interstitial fluid (ISF) surrounds the parenchymal cells of the brain and spinal cord while cerebrospinal fluid (CSF) fills the larger spaces within and around the CNS. Regulation of the composition and volume of these fluids is important for effective functioning of brain cells and is achieved by barriers that prevent free exchange between CNS and blood and by mechanisms that secrete fluid of controlled composition into the brain and distribute and reabsorb it. Structures associated with this regular fluid turnover include the choroid plexuses, brain capillaries comprising the blood-brain barrier, arachnoid villi and perineural spaces penetrating the cribriform plate. ISF flow, estimated from rates of removal of markers from the brain, has been thought to reflect rates of fluid secretion across the blood-brain barrier, although this has been questioned because measurements were made under barbiturate anaesthesia possibly affecting secretion and flow and because CSF influx to the parenchyma via perivascular routes may deliver fluid independently of blood-brain barrier secretion. Fluid secretion at the blood-brain barrier is provided by specific transporters that generate solute fluxes so creating osmotic gradients that force water to follow. Any flow due to hydrostatic pressures driving water across the barrier soon ceases unless accompanied by solute transport because water movements modify solute concentrations. CSF is thought to be derived primarily from secretion by the choroid plexuses. Flow rates measured using phase contrast magnetic resonance imaging reveal CSF movements to be more rapid and variable than previously supposed, even implying that under some circumstances net flow through the cerebral aqueduct may be reversed with net flow into the third and lateral ventricles. Such reversed flow requires there to be alternative sites for both generation and removal of CSF. Fluorescent tracer analysis has shown that fluid flow can occur from CSF into parenchyma along periarterial spaces. Whether this represents net fluid flow and whether there is subsequent flow through the interstitium and net flow out of the cortex via perivenous routes, described as glymphatic circulation, remains to be established. Modern techniques have revealed complex fluid movements within the brain. This review provides a critical evaluation of the data.
Keywords: Blood-brain barrier; Brain interstitial fluid; Cerebrospinal fluid; Choroid plexus; Convection; Diffusion; Filtration; Periarterial space; Phase contrast magnetic resonance imaging; Secretion.
Figures





References
-
- Woollam DHM, Millen JW. Perivascular spaces of the mammalian central nervous system. Biol Rev Camb Philos Soc. 1954;29:251–283. doi: 10.1111/j.1469-185X.1954.tb00597.x. - DOI
-
- Millen J, Woollam D. The Anatomy of the Cerebrospinal Fluid. London: Oxford University Press; 1962.
-
- Hayman LA, Weathers SW, Kirkpatrick JB. Atlas of cerebrospinal fluid spaces. In: Hayman LA, Hinck VC, editors. Clinical Brain Imaging: Normal Structure and Functional Anatomy. St. Louis: Mosby-Year Book; 1992. pp. 306–328.
-
- Cserr HF. Physiology of choroid plexus. Physiol Rev. 1971;51:273–311. - PubMed
-
- Welch K. The principles of physiology of the cerebrospinal fluid in relation to hydrocephalus including normal pressure hydrocephalus. Adv Neurol. 1975;13:247–332. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

