Routine blood cultures in the management of pyelonephritis in pregnancy for improving outcomes
- PMID: 25679346
- PMCID: PMC6823257
- DOI: 10.1002/14651858.CD009216.pub2
Routine blood cultures in the management of pyelonephritis in pregnancy for improving outcomes
Abstract
Background: Pyelonephritis is a type of urinary tract infection (UTI) that affects the upper urinary tract and kidneys, and is one of the most common conditions for hospitalisation among pregnant women, aside from delivery. Samples of urine and blood are obtained and used for cultures as part of the diagnosis and management of the condition. Acute pyelonephritis requires hospitalisation with intravenous administration of antimicrobial agents. Several studies have questioned the necessity of obtaining blood cultures in addition to urine cultures, citing cost and questioning whether blood testing is superfluous. Pregnant women with bacteraemia require a change in the initial empirical treatment based on the blood culture. However, these cases are not common, and represent approximately 15% to 20% of cases. It is unclear whether blood cultures are essential for the effective management of the condition.
Objectives: To assess the effectiveness of routine blood cultures to improve health outcomes in the management of pyelonephritis in pregnant women.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register without language or date restrictions (31 December 2014).
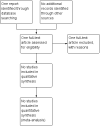
Selection criteria: Randomised controlled trials and quasi-randomised trials comparing outcomes among pregnant women with pyelonephritis who received initial management with or without blood cultures. Cluster-randomised trials were eligible for inclusion in this review but none were identified. Clinical trials using a cross-over design were not eligible for inclusion.
Data collection and analysis: Two review authors independently assessed one trial report for inclusion.
Main results: We identified one trial report but this was excluded. No clinical trials met the inclusion criteria for this review.
Authors' conclusions: There are no large-scale randomised controlled trials to assess outcomes in the management of pyelonephritis in pregnancy with or without blood cultures. Randomised controlled trials are needed to evaluate the effectiveness of managing pyelonephritis in pregnant women with or without blood cultures, and to assess any adverse outcomes as well as the cost-effectiveness of excluding blood cultures from treatment.
Conflict of interest statement
None known.
Update of
References
References to studies excluded from this review
Dozier 1997 {published data only}
-
- Dozier B, Nathan L. A prospective evaluation of the utility of blood cultures in treating antepartum pyelonephritis. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S24.
Additional references
Al‐Hasan 2009
Al‐Hasan 2010
Benenson 2007
-
- Benenson RS, Kepner AM, Pyle DN 2nd, Cavanaugh S. Selective use of blood cultures in emergency department pneumonia patients. Journal of Emergency Medicine 2007;33(1):1‐8. - PubMed
Cham 2009
-
- Cham G, Yan S, Hoon HB, Seow E. Predicting positive blood cultures in patients presenting with pneumonia at an emergency department in Singapore. Annals of Academic Medicine in Singapore 2009;38:508‐14. - PubMed
Chen 2006
-
- Chen Y, Nitzan O, Saliba W, Chazan B, Colodner R, Raz R. Are blood cultures necessary in the management of women with complicated pyelonephritis?. Journal of Infection 2006;53(4):235‐40. [PUBMED: 16434102] - PubMed
Cunningham 1973
-
- Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: a clinical review. Obstetrics & Gynecology 1973;42:112‐7. - PubMed
Cunningham 2009
-
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong KY. Williams Obstetrics. McGraw‐Hill Professional, 2009.
EARSS 2008
-
- European Antimicrobial Resistance Surveillance System (EARSS). European Antimicrobial Resistance Surveillance System Annual Report 2008. EARSS, 2008.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2005
-
- Hill JB, Sheffield JS, McIntire DD, Wendel GD Jr. Acute pyelonephritis in pregnancy. Obstetrics & Gynecology 2005;105:18‐23. - PubMed
Hsueh 2010
-
- Hsueh PR, Badal RE, Hawser SP, Hoban DJ, Bouchillon SK, Ni Y, et al. Epidemiology and antimicrobial susceptibility profiles of aerobic and facultative Gram‐negative bacilli isolated from patients with intra‐abdominal infections in the Asia‐Pacific region: 2008 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). International Journal of Antimicrobial Agents 2010;36:408‐14. - PubMed
Islam 2009
-
- Islam M, Yoshida S. Women are still deprived of access to lifesaving essential and emergency obstetric care. International Journal of Gynecology & Obstetrics 2009;106:120‐4. - PubMed
Jacobsson 2002
-
- Jacobsson B, Hagberg G, Hagberg B, Ladfors L, Niklasson A, Hagberg H. Cerebral palsy in preterm infants: a population‐based case‐control study of antenatal and intrapartal risk factors. Acta Paediatrica 2002;91:946‐51. - PubMed
Kongnyuy 2009
-
- Kongnyuy E, Hofman J, Mlava G, Mhango C, Broek N. Availability, utilisation and quality of basic and comprehensive emergency obstetric care services in Malawi. Maternal and Child Health Journal 2009;13:687‐94. - PubMed
Lang 1996
-
- Lang JM, Lieberman E, Cohen A. A comparison of risk factors for preterm labor and term small‐for‐gestation‐age birth. Epidemiology 1996;7:369‐76. - PubMed
Mabie 1997
-
- Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstetrics & Gynecology 1997;90:553‐61. - PubMed
MacMillan 1991
-
- MacMillan MC, Grimes DA. The limited usefulness of urine and blood cultures in treating pyelonephritis in pregnancy. Obstetrics & Gynecology 1991;78:745‐8. - PubMed
Masinde 2009
-
- Masinde A, Gumodoka B, Kilonzo A, Mshana SE. Prevalence of urinary tract infection among pregnant women at Bugando Medical Centre, Mwanza, Tanzania. Tanzanian Journal of Health Research 2009;11:154‐9. - PubMed
Millar 2003
-
- Millar LK, Debuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. Journal of Perinatal Medicine 2003;31:41‐6. - PubMed
Nicolle 2005
-
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clinical Infectious Diseases 2005;40:643‐54. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanchez‐Ramos 1995
-
- Sanchez‐Ramos L, McAlpine KJ, Adair CD, Kaunitz AM, Delke I, Briones DK. Pyelonephritis in pregnancy: once‐a‐day ceftriaxone versus multiple doses of cefazolin: a randomized, double‐blind trial. American Journal of Obstetrics and Gynecology 1995;172:129‐33. - PubMed
Scott 1997
-
- Scott CL, Chavez GF, Atrash HK, Taylor DJ, Shah RS, Rowley D. Hospitalizations for severe complications of pregnancy, 1987‐1992. Obstetrics & Gynecology 1997;90:225‐9. - PubMed
Shanarr 2008
-
- Shanarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. European Journal of Clinical Investigation 2008;38:50‐6. - PubMed
Smaill 2007
Solomkin 2010
-
- Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clinical Infectious Diseases 2010;50:133‐64. - PubMed
Stoneking 2013
-
- Stoneking LR, Patanwala AE, Winkler JP, Fiorello AB, Lee ES, Olson DP, et al. Would earlier microbe identification alter antibiotic therapy in bacteremic emergency department patients?. Journal of Emergency Medicine 2013;44:1‐8. - PubMed
Wing 2000
-
- Wing DA, Park AS, DeBuque L, Millar LK. Limited clinical utility of blood and urine cultures in the treatment of acute pyelonephritis during pregnancy. American Journal of Obstetrics and Gynecology 2000;182:1437‐40. - PubMed
Wing 2014
-
- Wing DA, Fassett MJ, Getahun D. Acute pyelonephritis in pregnancy: an 18‐year retrospective analysis. American Journal of Obstetrics and Gynecology 2014;210(3):219.e1‐219.e6. [PUBMED: 24100227] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous