Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: detection of specific antibody anti-gp43 and specific antigen gp43
- PMID: 25679976
- PMCID: PMC4334539
- DOI: 10.1371/journal.pntd.0003516
Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: detection of specific antibody anti-gp43 and specific antigen gp43
Abstract
Background: Paracoccidioidomycosis (PCM) is a life-threatening systemic disease and is a neglected public health problem in many endemic regions of Latin America. Though several diagnostic methods are available, almost all of them present with some limitations.
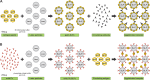
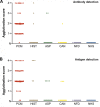
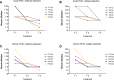
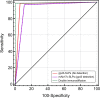
Method/principle findings: A latex immunoassay using sensitized latex particles (SLPs) with gp43 antigen, the immunodominant antigen of Paracoccidioides brasiliensis, or the monoclonal antibody mAb17c (anti-gp43) was evaluated for antibody or antigen detection in sera, cerebrospinal fluid (CSF), and bronchoalveolar lavage (BAL) from patients with PCM due to P. brasiliensis. The gp43-SLPs performed optimally to detect specific antibodies with high levels of sensitivity (98.46%, 95% CI 91.7-100.0), specificity (93.94%, 95% CI 87.3-97.7), and positive (91.4%) and negative (98.9%) predictive values. In addition, we propose the use of mAb17c-SLPs to detect circulating gp43, which would be particularly important in patients with immune deficiencies who fail to produce normal levels of immunoglobulins, achieving good levels of sensitivity (96.92%, 95% CI 89.3-99.6), specificity (88.89%, 95% CI 81.0-94.3), and positive (85.1%) and negative (97.8%) predictive values. Very good agreement between latex tests and double immune diffusion was observed for gp43-SLPs (k = 0.924) and mAb17c-SLPs (k = 0.850), which reinforces the usefulness of our tests for the rapid diagnosis of PCM in less than 10 minutes. Minor cross-reactivity occurred with sera from patients with other fungal infections. We successfully detected antigens and antibodies from CSF and BAL samples. In addition, the latex test was useful for monitoring PCM patients receiving therapy.
Conclusions/significance: The high diagnostic accuracy, low cost, reduced assay time, and simplicity of this new latex test offer the potential to be commercialized and makes it an attractive diagnostic assay for use not only in clinics and medical mycology laboratories, but mainly in remote locations with limited laboratory infrastructure and/or minimally trained community health workers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Detection of circulating gp43 antigen in serum, cerebrospinal fluid, and bronchoalveolar lavage fluid of patients with paracoccidioidomycosis.J Clin Microbiol. 2003 Aug;41(8):3675-80. doi: 10.1128/JCM.41.8.3675-3680.2003. J Clin Microbiol. 2003. PMID: 12904374 Free PMC article.
-
Serology of paracoccidioidomycosis.Mycopathologia. 2008 Apr-May;165(4-5):289-302. doi: 10.1007/s11046-007-9060-5. Mycopathologia. 2008. PMID: 18777635 Review.
-
Diagnosis of neuroparacoccidioidomycosis by detection of circulating antigen and antibody in cerebrospinal fluid.J Clin Microbiol. 2005 Sep;43(9):4680-3. doi: 10.1128/JCM.43.9.4680-4683.2005. J Clin Microbiol. 2005. PMID: 16145126 Free PMC article.
-
Diagnosis of Paracoccidioidomycosis by detection of antigen and antibody in bronchoalveolar lavage fluids.Clin Vaccine Immunol. 2006 Dec;13(12):1363-6. doi: 10.1128/CVI.00239-06. Epub 2006 Oct 11. Clin Vaccine Immunol. 2006. PMID: 17035512 Free PMC article.
-
Advances and challenges in paracoccidioidomycosis serology caused by Paracoccidioides species complex: an update.Diagn Microbiol Infect Dis. 2016 Jan;84(1):87-94. doi: 10.1016/j.diagmicrobio.2015.06.004. Epub 2015 Jun 12. Diagn Microbiol Infect Dis. 2016. PMID: 26494541 Review.
Cited by
-
Paracoccidioides and Paracoccidioidomycosis in the 21st Century.Mycopathologia. 2023 Apr;188(1-2):129-133. doi: 10.1007/s11046-022-00704-y. Epub 2023 Jan 12. Mycopathologia. 2023. PMID: 36633737 Review.
-
Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment.J Fungi (Basel). 2022 Oct 18;8(10):1098. doi: 10.3390/jof8101098. J Fungi (Basel). 2022. PMID: 36294662 Free PMC article. Review.
-
A New Duplex PCR-Assay for the Detection and Identification of Paracoccidioides Species.J Fungi (Basel). 2021 Feb 26;7(3):169. doi: 10.3390/jof7030169. J Fungi (Basel). 2021. PMID: 33652623 Free PMC article.
-
Clinical and epidemiological features of paracoccidioidomycosis due to Paracoccidioides lutzii.PLoS Negl Trop Dis. 2019 Jun 4;13(6):e0007437. doi: 10.1371/journal.pntd.0007437. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31163028 Free PMC article.
-
Standardization and validation of Dot-ELISA assay for Paracoccidioides brasiliensis antibody detection.J Venom Anim Toxins Incl Trop Dis. 2017 Feb 15;23:11. doi: 10.1186/s40409-017-0101-3. eCollection 2017. J Venom Anim Toxins Incl Trop Dis. 2017. PMID: 28239394 Free PMC article.
References
-
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, et al. (2007) Control of neglected tropical diseases. N Engl J Med 357: 1018–1027. - PubMed
-
- Shikanai-Yasuda MA, Telles Filho Fde Q, Mendes RP, Colombo AL, Moretti ML (2006) Guidelines in paracoccidioidomycosis. Rev Soc Bras Med Trop 39: 297–310. - PubMed
-
- Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, et al. (2006) Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol 23: 65–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

