Combined liquid chromatography-tandem mass spectrometry analysis of progesterone metabolites
- PMID: 25680188
- PMCID: PMC4332660
- DOI: 10.1371/journal.pone.0117984
Combined liquid chromatography-tandem mass spectrometry analysis of progesterone metabolites
Abstract
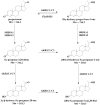
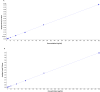
Progesterone has a number of important functions throughout the human body. While the roles of progesterone are well known, the possible actions and implications of progesterone metabolites in different tissues remain to be determined. There is a growing body of evidence that these metabolites are not inactive, but can have significant biological effects, as anesthetics, anxiolytics and anticonvulsants. Furthermore, they can facilitate synthesis of myelin components in the peripheral nervous system, have effects on human pregnancy and onset of labour, and have a neuroprotective role. For a better understanding of the functions of progesterone metabolites, improved analytical methods are essential. We have developed a combined liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for detection and quantification of progesterone and 16 progesterone metabolites that has femtomolar sensitivity and good reproducibility in a single chromatographic run. MS/MS analyses were performed in positive mode and under constant electrospray ionization conditions. To increase the sensitivity, all of the transitions were recorded using the Scheduled MRM algorithm. This LC-MS/MS method requires small sample volumes and minimal sample preparation, and there is no need for derivatization. Here, we show the application of this method for evaluation of progesterone metabolism in the HES endometrial cell line. In HES cells, the metabolism of progesterone proceeds mainly to (20S)-20-hydroxy-pregn-4-ene-3-one, (20S)-20-hydroxy-5α-pregnane-3-one and (20S)-5α-pregnane-3α,20-diol. The investigation of possible biological effects of these metabolites on the endometrium is currently undergoing.
Conflict of interest statement
Figures




References
-
- Graham JD, Clarke CL (1997) Physiological action of progesterone in target tissues. Endocr Rev 18: 502–519. - PubMed
-
- Ismail PM, Amato P, Soyal SM, DeMayo FJ, Conneely OM, et al. (2003) Progesterone involvement in breast development and tumorigenesis-as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids 68: 779–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

