Tumor necrosis factor superfamily 14 (LIGHT) controls thymic stromal lymphopoietin to drive pulmonary fibrosis
- PMID: 25680454
- PMCID: PMC4532661
- DOI: 10.1016/j.jaci.2014.12.1936
Tumor necrosis factor superfamily 14 (LIGHT) controls thymic stromal lymphopoietin to drive pulmonary fibrosis
Abstract
Background: Pulmonary fibrosis is characterized by excessive accumulation of collagen and α-smooth muscle actin in the lung. The key molecules that promote these phenotypes are of clinical interest.
Objectives: Thymic stromal lymphopoietin (TSLP) has been found at high levels in patients with asthma and idiopathic pulmonary fibrosis, and TSLP has been proposed as a primary driver of lung fibrotic disease. We asked whether tumor necrosis factor superfamily protein 14 (TNFSF14) (aka LIGHT) controls TSLP production to initiate fibrosis.
Methods: Expression of TSLP and initiation of pulmonary fibrosis induced by bleomycin were assessed in mice deficient in LIGHT. The ability of recombinant LIGHT, given intratracheally to naive mice, to promote TSLP and fibrosis was also determined.
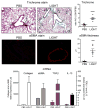
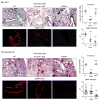
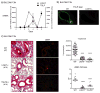
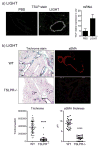
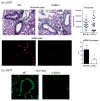
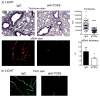
Results: Genetic deletion of LIGHT abolished lung TSLP expression driven by bleomycin, accompanied by near-complete absence of accumulation of lung collagen and α-smooth muscle actin. Furthermore, recombinant LIGHT administered in vivo induced lung expression of TSLP in the absence of other inflammatory stimuli, and strikingly reproduced the primary features of bleomycin-driven disease in a TSLP-dependent manner. Blockade of LIGHT binding to either of its receptors, herpes virus entry mediator and lymphotoxin beta receptor, inhibited clinical symptoms of pulmonary fibrosis, and correspondingly both receptors were found on human bronchial epithelial cells, a primary source of TSLP. Moreover, LIGHT induced TSLP directly in human bronchial epithelial cells and synergized with IL-13 and TGF-β in vivo to promote TSLP in the lungs and drive fibrosis.
Conclusions: These results show that LIGHT is a profibrogenic cytokine that may be a key driver of TSLP production during the initiation and development of lung fibrotic disease.
Keywords: HVEM; IPF; LIGHT; Pulmonary fibrosis; TNFSF14; TSLP; asthma; bronchial epithelial cell.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Thannickal VJ, Toews GB, White eS, Lynch P, Jr, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. - PubMed
-
- Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010 Feb 2;152(3):159–66. [Research Support, Non-U.S. Gov’t Review] - PubMed
-
- Boin F, Wigley F. Connective tissue diseases: Immunosuppressive therapy in SSc: what is the target? Nat Rev Rheumatol. 2009 Jul;5(7):357–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

