Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424
- PMID: 25680596
- PMCID: PMC4329190
- DOI: 10.1016/j.ijrobp.2014.11.012
Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424
Abstract
Purpose: Radiation Therapy Oncology Group (RTOG) 0424 was a phase 2 study of a high-risk low-grade glioma (LGG) population who were treated with temozolomide (TMZ) and radiation therapy (RT), and outcomes were compared to those of historical controls. This study was designed to detect a 43% increase in median survival time (MST) from 40.5 to 57.9 months and a 20% improvement in 3-year overall survival (OS) rate from 54% to 65% at a 10% significance level (1-sided) and 96% power.
Methods and materials: Patients with LGGs with 3 or more risk factors for recurrence (age ≥40 years, astrocytoma histology, bihemispherical tumor, preoperative tumor diameter of ≥6 cm, or a preoperative neurological function status of >1) were treated with RT (54 Gy in 30 fractions) and concurrent and adjuvant TMZ.
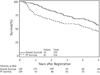
Results: From 2005 to 2009, 129 evaluable patients (75 males and 54 females) were accrued. Median age was 49 years; 91% had a Zubrod score of 0 or 1; and 69%, 25%, and 6% of patients had 3, 4, and 5 risk factors, respectively. Patients had median and minimum follow-up examinations of 4.1 years and 3 years, respectively. The 3-year OS rate was 73.1% (95% confidence interval: 65.3%-80.8%), which was significantly improved compared to that of prespecified historical control values (P<.001). Median survival time has not yet been reached. Three-year progression-free survival was 59.2%. Grades 3 and 4 adverse events occurred in 43% and 10% of patients, respectively. One patient died of herpes encephalitis.
Conclusions: The 3-year OS rate of 73.1% for RTOG 0424 high-risk LGG patients is higher than that reported for historical controls (P<.001) and the study-hypothesized rate of 65%.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: none.
Figures
References
-
- Quinn J, Reardon D, Friedman A, et al. Phase II trial of temozolomide in patients with progressive or recurrent low-grade glioma. J Clin Oncol. 2003;21:646–651. - PubMed
-
- van den Bent M, Chinot O, Boogerd W, et al. EORTC Study 26972: Second line chemotherapy with temozolomide in recurrent oligodendroglioma after PCV (procarbazine, CCNU, vincristine) chemotherapy: EORTC phase II study 26. Ann Oncol. 2003;14:599–602. - PubMed
-
- Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009;15:330–337. - PubMed
-
- Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurol. 2007;68:1831–1836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical