Food allergies: the basics
- PMID: 25680669
- PMCID: PMC4414527
- DOI: 10.1053/j.gastro.2015.02.006
Food allergies: the basics
Abstract
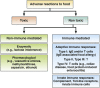
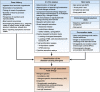
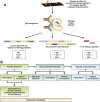
IgE-associated food allergy affects approximately 3% of the population and has severe effects on the daily life of patients-manifestations occur not only in the gastrointestinal tract but also affect other organ systems. Birth cohort studies have shown that allergic sensitization to food allergens develops early in childhood. Mechanisms of pathogenesis include cross-linking of mast cell- and basophil-bound IgE and immediate release of inflammatory mediators, as well as late-phase and chronic allergic inflammation, resulting from T-cell, basophil, and eosinophil activation. Researchers have begun to characterize the molecular features of food allergens and have developed chip-based assays for multiple allergens. These have provided information about cross-reactivity among different sources of food allergens, identified disease-causing food allergens, and helped us to estimate the severity and types of allergic reactions in patients. Importantly, learning about the structure of disease-causing food allergens has allowed researchers to engineer synthetic and recombinant vaccines.
Keywords: Allergen; Diagnosis; IgE; IgE-Associated Food Allergy; Immunotherapy; Multiallergen Test; Therapy.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Metcalfe D.D., Sampson H.A., Simon R.A. 3rd ed. Blackwell Science; Malden, MA: 2003. Food allergy: adverse reactions to foods and food additives.
-
- Bischoff S.C., Sellge G. 3rd ed. Blackwell Science; Malden, MA: 2003. Immune mechanisms in food-induced disease; pp. 14–37. (Food allergy: adverse reactions to foods and food additives).
-
- Sicherer S.H., Sampson H.A. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. - PubMed
-
- Longo G., Berti I., Burks A.W., et al. IgE-mediated food allergy in children. Lancet. 2013;382:1656–1664. - PubMed
-
- De Silva D., Geromi M., Panesar S.S., et al. Acute and long-term management of food allergy: systematic review. Allergy. 2014;69:159–167. - PubMed
Supplementary References
-
- Katelaris C.H., Lee B.W., Potter P.C., et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012;42:186–207. - PubMed
-
- Strobel S., Mowat A.M. Oral tolerance and allergic responses to food proteins. Curr Opin Allergy Clin Immunol. 2006;6:207–213. - PubMed
-
- Chehade M., Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

