Novel tools for genetic manipulation of follicle stem cells in the Drosophila ovary reveal an integrin-dependent transition from quiescence to proliferation
- PMID: 25680813
- PMCID: PMC4391569
- DOI: 10.1534/genetics.114.173617
Novel tools for genetic manipulation of follicle stem cells in the Drosophila ovary reveal an integrin-dependent transition from quiescence to proliferation
Abstract
In many tissues, the presence of stem cells is inferred by the capacity of the tissue to maintain homeostasis and undergo repair after injury. Isolation of self-renewing cells with the ability to generate the full array of cells within a given tissue strongly supports this idea, but the identification and genetic manipulation of individual stem cells within their niche remain a challenge. Here we present novel methods for marking and genetically altering epithelial follicle stem cells (FSCs) within the Drosophila ovary. Using these new tools, we define a sequential multistep process that comprises transitioning of FSCs from quiescence to proliferation. We further demonstrate that integrins are cell-autonomously required within FSCs to provide directional signals that are necessary at each step of this process. These methods may be used to define precise roles for specific genes in the sequential events that occur during FSC division after a period of quiescence.
Keywords: adhesion; follicle stem cell; germarium; integrin; quiescence.
Copyright © 2015 by the Genetics Society of America.
Figures


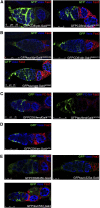
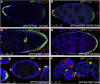


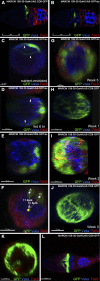



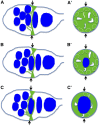
References
-
- Assa-Kunik E., Torres I. L., Schejter E. D., Johnston D. S., Shilo B. Z., 2007. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 134: 1161–1169. - PubMed
-
- Bolívar J., Pearson J., López-Onieva L., González-Reyes A., 2006. Genetic dissection of a stem cell niche: The case of the Drosophila ovary. Dev. Dyn. 235: 2969–2979. - PubMed
-
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. - PubMed
-
- Cabrera G. R., Godt D., Fang P. Y., Couderc J. L., Laski F. A., 2002. Expression pattern of Gal4 enhancer trap insertions into the bric à brac locus generated by P element replacement. Genesis 34: 62–65. - PubMed
-
- Casanueva M. O., Ferguson E. L., 2004. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development 131: 1881–1890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

