The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins
- PMID: 25681191
- PMCID: PMC4375313
- DOI: 10.1128/AEM.00193-15
The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins
Abstract
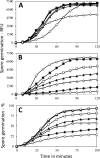
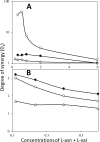
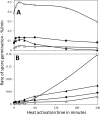
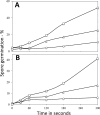
Nutrient germination of spores of Bacillus species occurs through germinant receptors (GRs) in spores' inner membrane (IM) in a process stimulated by sublethal heat activation. Bacillus subtilis spores maximum germination rates via different GRs required different 75 °C heat activation times: 15 min for l-valine germination via the GerA GR and 4 h for germination with the L-asparagine-glucose-fructose-K(+) mixture via the GerB and GerK GRs, with GerK requiring the most heat activation. In some cases, optimal heat activation decreased nutrient concentrations for half-maximal germination rates. Germination of spores via various GRs by high pressure (HP) of 150 MPa exhibited heat activation requirements similar to those of nutrient germination, and the loss of the GerD protein, required for optimal GR function, did not eliminate heat activation requirements for maximal germination rates. These results are consistent with heat activation acting primarily on GRs. However, (i) heat activation had no effects on GR or GerD protein conformation, as probed by biotinylation by an external reagent; (ii) spores prepared at low and high temperatures that affect spores' IM properties exhibited large differences in heat activation requirements for nutrient germination; and (iii) spore germination by 550 MPa of HP was also affected by heat activation, but the effects were relatively GR independent. The last results are consistent with heat activation affecting spores' IM and only indirectly affecting GRs. The 150- and 550-MPa HP germinations of Bacillus amyloliquefaciens spores, a potential surrogate for Clostridium botulinum spores in HP treatments of foods, were also stimulated by heat activation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Spore Heat Activation Requirements and Germination Responses Correlate with Sequences of Germinant Receptors and with the Presence of a Specific spoVA2mob Operon in Foodborne Strains of Bacillus subtilis.Appl Environ Microbiol. 2017 Mar 17;83(7):e03122-16. doi: 10.1128/AEM.03122-16. Print 2017 Apr 1. Appl Environ Microbiol. 2017. PMID: 28130296 Free PMC article.
-
Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis.J Bacteriol. 2003 Apr;185(8):2457-64. doi: 10.1128/JB.185.8.2457-2464.2003. J Bacteriol. 2003. PMID: 12670969 Free PMC article.
-
Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor.J Bacteriol. 2006 Jan;188(1):28-36. doi: 10.1128/JB.188.1.28-36.2006. J Bacteriol. 2006. PMID: 16352818 Free PMC article.
-
Germination of spores of Bacillus species: what we know and do not know.J Bacteriol. 2014 Apr;196(7):1297-305. doi: 10.1128/JB.01455-13. Epub 2014 Jan 31. J Bacteriol. 2014. PMID: 24488313 Free PMC article. Review.
-
Recent progress in proteins regulating the germination of Bacillus subtilis spores.J Bacteriol. 2025 Feb 20;207(2):e0028524. doi: 10.1128/jb.00285-24. Epub 2025 Jan 8. J Bacteriol. 2025. PMID: 39772627 Free PMC article. Review.
Cited by
-
Changes in Bacillus Spore Small Molecules, rRNA, Germination, and Outgrowth after Extended Sublethal Exposure to Various Temperatures: Evidence that Protein Synthesis Is Not Essential for Spore Germination.J Bacteriol. 2016 Nov 18;198(24):3254-3264. doi: 10.1128/JB.00583-16. Print 2016 Dec 15. J Bacteriol. 2016. PMID: 27645383 Free PMC article.
-
Spore Heat Activation Requirements and Germination Responses Correlate with Sequences of Germinant Receptors and with the Presence of a Specific spoVA2mob Operon in Foodborne Strains of Bacillus subtilis.Appl Environ Microbiol. 2017 Mar 17;83(7):e03122-16. doi: 10.1128/AEM.03122-16. Print 2017 Apr 1. Appl Environ Microbiol. 2017. PMID: 28130296 Free PMC article.
-
Influence of Sporulation Temperature on Germination and Growth of B. weihenstephanensis Strains in Specific Nutrients and in an Extended Shelf-Life Refrigerated Matrix Under Commercial Pasteurization and Storage Conditions.Foods. 2024 Oct 28;13(21):3434. doi: 10.3390/foods13213434. Foods. 2024. PMID: 39517218 Free PMC article.
-
Recent Progress in the Synergistic Bactericidal Effect of High Pressure and Temperature Processing in Fruits and Vegetables and Related Kinetics.Foods. 2022 Nov 18;11(22):3698. doi: 10.3390/foods11223698. Foods. 2022. PMID: 36429290 Free PMC article. Review.
-
Effects of High-Pressure Treatment on Spores of Clostridium Species.Appl Environ Microbiol. 2016 Aug 15;82(17):5287-97. doi: 10.1128/AEM.01363-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27316969 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

