Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA
- PMID: 25681199
- PMCID: PMC4498659
- DOI: 10.1016/j.jpeds.2014.12.069
Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA
Abstract
Objectives: To test the hypothesis that somatic phosphatidylinositol-4,5-bisphospate 3-kinase, catalytic subunit alpha (PIK3CA) mutations would be found in patients with more common disorders including isolated lymphatic malformation (LM) and Klippel-Trenaunay syndrome (KTS).
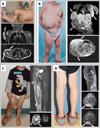
Study design: We used next generation sequencing, droplet digital polymerase chain reaction, and single molecule molecular inversion probes to search for somatic PIK3CA mutations in affected tissue from patients seen at Boston Children's Hospital who had an isolated LM (n = 17), KTS (n = 21), fibro-adipose vascular anomaly (n = 8), or congenital lipomatous overgrowth with vascular, epidermal, and skeletal anomalies syndrome (n = 33), the disorder for which we first identified somatic PIK3CA mutations. We also screened 5 of the more common PIK3CA mutations in a second cohort of patients with LM (n = 31) from Seattle Children's Hospital.
Results: Most individuals from Boston Children's Hospital who had isolated LM (16/17) or LM as part of a syndrome, such as KTS (19/21), fibro-adipose vascular anomaly (5/8), and congenital lipomatous overgrowth with vascular, epidermal, and skeletal anomalies syndrome (31/33) were somatic mosaic for PIK3CA mutations, with 5 specific PIK3CA mutations accounting for ∼ 80% of cases. Seventy-four percent of patients with LM from Seattle Children's Hospital also were somatic mosaic for 1 of 5 specific PIK3CA mutations. Many affected tissue specimens from both cohorts contained fewer than 10% mutant cells.
Conclusions: Somatic PIK3CA mutations are the most common cause of isolated LMs and disorders in which LM is a component feature. Five PIK3CA mutations account for most cases. The search for causal mutations requires sampling of affected tissues and techniques that are capable of detecting low-level somatic mosaicism because the abundance of mutant cells in a malformed tissue can be low.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

