The role of the plant-specific ALTERED XYLOGLUCAN9 protein in Arabidopsis cell wall polysaccharide O-acetylation
- PMID: 25681330
- PMCID: PMC4378174
- DOI: 10.1104/pp.114.256479
The role of the plant-specific ALTERED XYLOGLUCAN9 protein in Arabidopsis cell wall polysaccharide O-acetylation
Abstract
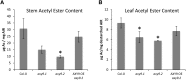
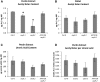
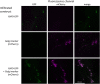
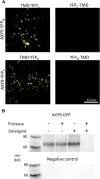
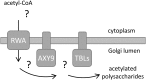
A mutation in the ALTERED XYLOGLUCAN9 (AXY9) gene was found to be causative for the decreased xyloglucan acetylation phenotype of the axy9.1 mutant, which was identified in a forward genetic screen for Arabidopsis (Arabidopsis thaliana) mutants. The axy9.1 mutant also exhibits decreased O-acetylation of xylan, implying that the AXY9 protein has a broad role in polysaccharide acetylation. An axy9 insertional mutant exhibits severe growth defects and collapsed xylem, demonstrating the importance of wall polysaccharide O-acetylation for normal plant growth and development. Localization and topological experiments indicate that the active site of the AXY9 protein resides within the Golgi lumen. The AXY9 protein appears to be a component of the plant cell wall polysaccharide acetylation pathway, which also includes the REDUCED WALL ACETYLATION and TRICHOME BIREFRINGENCE-LIKE proteins. The AXY9 protein is distinct from the TRICHOME BIREFRINGENCE-LIKE proteins, reported to be polysaccharide acetyltransferases, but does share homology with them and other acetyltransferases, suggesting that the AXY9 protein may act to produce an acetylated intermediate that is part of the O-acetylation pathway.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures



Similar articles
-
O-acetylation of Arabidopsis hemicellulose xyloglucan requires AXY4 or AXY4L, proteins with a TBL and DUF231 domain.Plant Cell. 2011 Nov;23(11):4041-53. doi: 10.1105/tpc.111.091728. Epub 2011 Nov 15. Plant Cell. 2011. PMID: 22086088 Free PMC article.
-
TBL3 and TBL31, Two Arabidopsis DUF231 Domain Proteins, are Required for 3-O-Monoacetylation of Xylan.Plant Cell Physiol. 2016 Jan;57(1):35-45. doi: 10.1093/pcp/pcv172. Epub 2015 Nov 9. Plant Cell Physiol. 2016. PMID: 26556650
-
Xyloglucan O-acetyltransferases from Arabidopsis thaliana and Populus trichocarpa catalyze acetylation of fucosylated galactose residues on xyloglucan side chains.Planta. 2018 Nov;248(5):1159-1171. doi: 10.1007/s00425-018-2972-0. Epub 2018 Aug 6. Planta. 2018. PMID: 30083810
-
Plant Cell Wall Polysaccharide O-Acetyltransferases.Plants (Basel). 2024 Aug 19;13(16):2304. doi: 10.3390/plants13162304. Plants (Basel). 2024. PMID: 39204739 Free PMC article. Review.
-
Balanced Xylan Acetylation is the Key Regulator of Plant Growth and Development, and Cell Wall Structure and for Industrial Utilization.Int J Mol Sci. 2020 Oct 23;21(21):7875. doi: 10.3390/ijms21217875. Int J Mol Sci. 2020. PMID: 33114198 Free PMC article. Review.
Cited by
-
Defense Mechanisms of Cotton Fusarium and Verticillium Wilt and Comparison of Pathogenic Response in Cotton and Humans.Int J Mol Sci. 2022 Oct 13;23(20):12217. doi: 10.3390/ijms232012217. Int J Mol Sci. 2022. PMID: 36293072 Free PMC article. Review.
-
A combination of genome-wide association study and transcriptome analysis in leaf epidermis identifies candidate genes involved in cuticular wax biosynthesis in Brassica napus.BMC Plant Biol. 2020 Oct 6;20(1):458. doi: 10.1186/s12870-020-02675-y. BMC Plant Biol. 2020. PMID: 33023503 Free PMC article.
-
Genome-wide characterization of trichome birefringence-like genes provides insights into fiber yield improvement.Front Plant Sci. 2023 Mar 15;14:1127760. doi: 10.3389/fpls.2023.1127760. eCollection 2023. Front Plant Sci. 2023. PMID: 37008510 Free PMC article.
-
Secretion of Acetylxylan Esterase From Chlamydomonas reinhardtii Enables Utilization of Lignocellulosic Biomass as a Carbon Source.Front Bioeng Biotechnol. 2019 Feb 28;7:35. doi: 10.3389/fbioe.2019.00035. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 30873405 Free PMC article.
-
Monitoring Polysaccharide Dynamics in the Plant Cell Wall.Plant Physiol. 2018 Apr;176(4):2590-2600. doi: 10.1104/pp.17.01776. Epub 2018 Feb 27. Plant Physiol. 2018. PMID: 29487120 Free PMC article. Review.
References
-
- Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF (2004) GDSL family of serine esterases/lipases. Prog Lipid Res 43: 534–552 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

