Transforming Growth Factor β/activin signalling induces epithelial cell flattening during Drosophila oogenesis
- PMID: 25681395
- PMCID: PMC4359740
- DOI: 10.1242/bio.201410785
Transforming Growth Factor β/activin signalling induces epithelial cell flattening during Drosophila oogenesis
Abstract
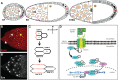
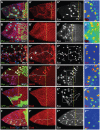
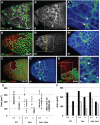
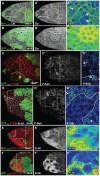
Although the regulation of epithelial morphogenesis is essential for the formation of tissues and organs in multicellular organisms, little is known about how signalling pathways control cell shape changes in space and time. In the Drosophila ovarian epithelium, the transition from a cuboidal to a squamous shape is accompanied by a wave of cell flattening and by the ordered remodelling of E-cadherin-based adherens junctions. We show that activation of the TGFβ pathway is crucial to determine the timing, the degree and the dynamic of cell flattening. Within these cells, TGFβ signalling controls cell-autonomously the formation of Actin filament and the localisation of activated Myosin II, indicating that internal forces are generated and used to remodel AJ and to promote cytoskeleton rearrangement. Our results also reveal that TGFβ signalling controls Notch activity and that its functions are partly executed through Notch. Thus, we demonstrate that the cells that undergo the cuboidal-to-squamous transition produce active cell-shaping mechanisms, rather than passively flattening in response to a global force generated by the growth of the underlying cells. Thus, our work on TGFβ signalling provides new insights into the mechanisms through which signal transduction cascades orchestrate cell shape changes to generate proper organ structure.
Keywords: Adherens junction; Epithelial cell flattening; TGFβ.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures


References
-
- Baker R., Schubiger G. (1996). Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development 122, 617–626. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

