Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys
- PMID: 25681412
- PMCID: PMC5014077
- DOI: 10.1093/brain/awv018
Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys
Abstract
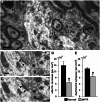
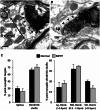
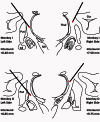
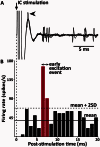
The striatum and the subthalamic nucleus are the main entry points for cortical information to the basal ganglia. Parkinson's disease affects not only the function, but also the morphological integrity of some of these inputs and their synaptic targets in the basal ganglia. Significant morphological changes in the cortico-striatal system have already been recognized in patients with Parkinson's disease and in animal models of the disease. To find out whether the primate cortico-subthalamic system is also subject to functionally relevant morphological alterations in parkinsonism, we used a combination of light and electron microscopy anatomical approaches and in vivo electrophysiological methods in monkeys rendered parkinsonian following chronic exposure to low doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). At the light microscopic level, the density of vesicular glutamate transporter 1-positive (i.e. cortico-subthalamic) profiles in the dorsolateral part of the subthalamic nucleus (i.e. its sensorimotor territory) was 26.1% lower in MPTP-treated parkinsonian monkeys than in controls. These results were confirmed by electron microscopy studies showing that the number of vesicular glutamate transporter 1-positive terminals and of axon terminals forming asymmetric synapses in the dorsolateral subthalamic nucleus was reduced by 55.1% and 27.9%, respectively, compared with controls. These anatomical findings were in line with in vivo electrophysiology data showing a 60% reduction in the proportion of pallidal neurons that responded to electrical stimulation of the cortico-subthalamic system in parkinsonian monkeys. These findings provide strong evidence for a partial loss of the hyperdirect cortico-subthalamic projection in MPTP-treated parkinsonian monkeys.
Keywords: Parkinson’s disease; cortico-subthalamic; hyperdirect; synaptic plasticity; vesicular glutamate transporter.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, et al. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage. 2011;55:1728–38. - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–20. - PubMed
-
- Bevan MD, Francis CM, Bolam JP. The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol. 1995;361:491–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

