Ubiquitin-associated domain-containing ubiquitin regulatory X (UBX) protein UBXN1 is a negative regulator of nuclear factor κB (NF-κB) signaling
- PMID: 25681446
- PMCID: PMC4400349
- DOI: 10.1074/jbc.M114.631689
Ubiquitin-associated domain-containing ubiquitin regulatory X (UBX) protein UBXN1 is a negative regulator of nuclear factor κB (NF-κB) signaling
Abstract
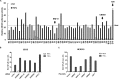
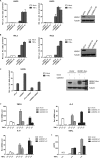
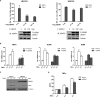
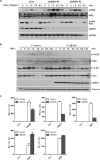
Excessive nuclear factor κB (NF-κB) activation should be precisely controlled as it contributes to multiple immune and inflammatory diseases. However, the negative regulatory mechanisms of NF-κB activation still need to be elucidated. Various types of polyubiquitin chains have proved to be involved in the process of NF-κB activation. Many negative regulators linked to ubiquitination, such as A20 and CYLD, inhibit IκB kinase activation in the NF-κB signaling pathway. To find new NF-κB signaling regulators linked to ubiquitination, we used a small scale siRNA library against 51 ubiquitin-associated domain-containing proteins and screened out UBXN1, which contained both ubiquitin-associated and ubiquitin regulatory X (UBX) domains as a negative regulator of TNFα-triggered NF-κB activation. Overexpression of UBXN1 inhibited TNFα-triggered NF-κB activation, although knockdown of UBXN1 had the opposite effect. UBX domain-containing proteins usually act as valosin-containing protein (VCP)/p97 cofactors. However, knockdown of VCP/p97 barely affected UBXN1-mediated NF-κB inhibition. At the same time, we found that UBXN1 interacted with cellular inhibitors of apoptosis proteins (cIAPs), E3 ubiquitin ligases of RIP1 in the TNFα receptor complex. UBXN1 competitively bound to cIAP1, blocked cIAP1 recruitment to TNFR1, and sequentially inhibited RIP1 polyubiquitination in response to TNFα. Therefore, our findings demonstrate that UBXN1 is an important negative regulator of the TNFα-triggered NF-κB signaling pathway by mediating cIAP recruitment independent of VCP/p97.
Keywords: Inflammation; NF-κB; Polyubiquitin Chain; Signal Transduction; Tumor Necrosis Factor (TNF); UBXN1; cIAPs.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Identification of F-box only protein 7 as a negative regulator of NF-kappaB signalling.J Cell Mol Med. 2012 Sep;16(9):2140-9. doi: 10.1111/j.1582-4934.2012.01524.x. J Cell Mol Med. 2012. PMID: 22212761 Free PMC article.
-
c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation.J Biol Chem. 2008 Sep 5;283(36):24295-9. doi: 10.1074/jbc.C800128200. Epub 2008 Jul 11. J Biol Chem. 2008. PMID: 18621737 Free PMC article.
-
Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination.Cell Death Dis. 2015 Jun 25;6(6):e1800. doi: 10.1038/cddis.2015.158. Cell Death Dis. 2015. PMID: 26111062 Free PMC article.
-
ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling.Biochem Pharmacol. 2009 Jul 15;78(2):105-14. doi: 10.1016/j.bcp.2009.02.009. Epub 2009 Feb 27. Biochem Pharmacol. 2009. PMID: 19464428 Review.
-
TNFR1 signaling kinetics: spatiotemporal control of three phases of IKK activation by posttranslational modification.Cell Signal. 2013 Aug;25(8):1654-64. doi: 10.1016/j.cellsig.2013.04.005. Epub 2013 Apr 21. Cell Signal. 2013. PMID: 23612498 Free PMC article. Review.
Cited by
-
PTRF/Cavin-1 as a Novel RNA-Binding Protein Expedites the NF-κB/PD-L1 Axis by Stabilizing lncRNA NEAT1, Contributing to Tumorigenesis and Immune Evasion in Glioblastoma.Front Immunol. 2022 Jan 6;12:802795. doi: 10.3389/fimmu.2021.802795. eCollection 2021. Front Immunol. 2022. PMID: 35069587 Free PMC article.
-
The Oncogenic Role of UBXN1 in Gastric Cancer Is Attributed to the METTL16-Mediated m6A Methylation and Histone Modifications.Cancer Med. 2025 Mar;14(6):e70772. doi: 10.1002/cam4.70772. Cancer Med. 2025. PMID: 40095756 Free PMC article.
-
Molecular Signatures of Fusion Proteins in Cancer.ACS Pharmacol Transl Sci. 2019 Mar 20;2(2):122-133. doi: 10.1021/acsptsci.9b00019. eCollection 2019 Apr 12. ACS Pharmacol Transl Sci. 2019. PMID: 32219217 Free PMC article.
-
Whole Transcriptome Sequencing Reveals Cancer-Related, Prognostically Significant Transcripts and Tumor-Infiltrating Immunocytes in Mantle Cell Lymphoma.Cells. 2022 Oct 27;11(21):3394. doi: 10.3390/cells11213394. Cells. 2022. PMID: 36359790 Free PMC article.
-
UBX Domain Protein 6 Positively Regulates JAK-STAT1/2 Signaling.J Immunol. 2021 Jun 1;206(11):2682-2691. doi: 10.4049/jimmunol.1901337. Epub 2021 May 21. J Immunol. 2021. PMID: 34021047 Free PMC article.
References
-
- Karin M. (2006) Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 - PubMed
-
- DiDonato J. A., Mercurio F., Karin M. (2012) NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 - PubMed
-
- Chen G., Goeddel D. V. (2002) TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 - PubMed
-
- Wajant H., Pfizenmaier K., Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

