Role of extracellular histones in the cardiomyopathy of sepsis
- PMID: 25681459
- PMCID: PMC4415013
- DOI: 10.1096/fj.14-268730
Role of extracellular histones in the cardiomyopathy of sepsis
Abstract
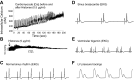
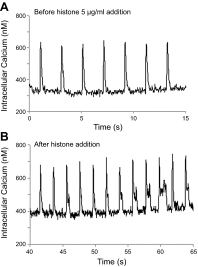
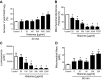
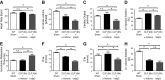
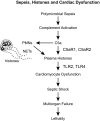
The purpose of this study was to define the relationship in polymicrobial sepsis (in adult male C57BL/6 mice) between heart dysfunction and the appearance in plasma of extracellular histones. Procedures included induction of sepsis by cecal ligation and puncture and measurement of heart function using echocardiogram/Doppler parameters. We assessed the ability of histones to cause disequilibrium in the redox status and intracellular [Ca(2+)]i levels in cardiomyocytes (CMs) (from mice and rats). We also studied the ability of histones to disturb both functional and electrical responses of hearts perfused with histones. Main findings revealed that extracellular histones appearing in septic plasma required C5a receptors, polymorphonuclear leukocytes (PMNs), and the Nacht-, LRR-, and PYD-domains-containing protein 3 (NLRP3) inflammasome. In vitro exposure of CMs to histones caused loss of homeostasis of the redox system and in [Ca(2+)]i, as well as defects in mitochondrial function. Perfusion of hearts with histones caused electrical and functional dysfunction. Finally, in vivo neutralization of histones in septic mice markedly reduced the parameters of heart dysfunction. Histones caused dysfunction in hearts during polymicrobial sepsis. These events could be attenuated by histone neutralization, suggesting that histones may be targets in the setting of sepsis to reduce cardiac dysfunction.
Keywords: NLRP3 inflammasome; cardiomyocytes; complement; echocardiogram/Doppler; polymicrobial sepsis.
© FASEB.
Figures


References
-
- Charpentier J., Luyt C. E., Fulla Y., Vinsonneau C., Cariou A., Grabar S., Dhainaut J. F., Mira J. P., Chiche J. D. (2004) Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit. Care Med. 32, 660–665 - PubMed
-
- Celes M. R., Prado C. M., Rossi M. A. (2013) Sepsis: going to the heart of the matter. Pathobiology 80, 70–86 - PubMed
-
- Rudiger A., Singer M. (2007) Mechanisms of sepsis-induced cardiac dysfunction. Crit. Care Med. 35, 1599–1608 - PubMed
-
- Parker M. M., Shelhamer J. H., Bacharach S. L., Green M. V., Natanson C., Frederick T. M., Damske B. A., Parrillo J. E. (1984) Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 100, 483–490 - PubMed
-
- Seong S. Y., Matzinger P. (2004) Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4, 469–478 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

