Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways
- PMID: 25681639
- PMCID: PMC4359098
- DOI: 10.1016/j.exger.2015.02.007
Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways
Abstract
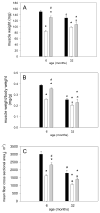
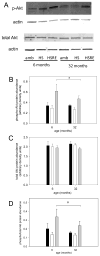
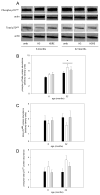
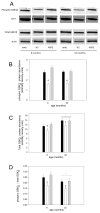
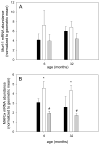
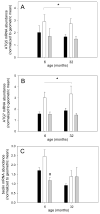
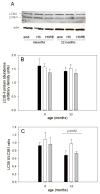
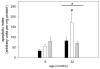
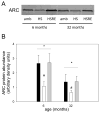
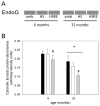
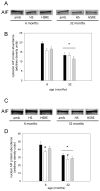
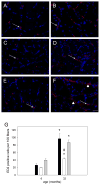
Skeletal muscle regrowth after atrophy is impaired in the aged and in this study we hypothesized that this can be explained by a blunted response of signaling pathways and cellular processes during reloading after hind limb suspension in muscles from old rats. Male Brown Norway Fisher 344 rats at 6 (young) and 32 (old) months of age were subjected to normal ambulatory conditions (amb), hind limb suspension for 14 days (HS), and HS followed by reloading through normal ambulation for 14 days (RE); soleus muscles were used for analysis of intracellular signaling pathways and cellular processes. Soleus muscle regrowth was blunted in old compared to young rats which coincided with a recovery of serum IGF-1 and IGFBP-3 levels in young but not old. However, the response to reloading for p-Akt, p-p70s6k and p-GSK3β protein abundance was similar between muscles from young and old rats, even though main effects for age indicate an increase in activation of this protein synthesis pathway in the aged. Similarly, MAFbx mRNA levels in soleus muscle from old rats recovered to the same extent as in the young, while Murf-1 was unchanged. mRNA abundance of autophagy markers Atg5 and Atg7 showed an identical response in muscle from old compared to young rats, but beclin did not. Autophagic flux was not changed at either age at the measured time point. Apoptosis was elevated in soleus muscle from old rats particularly with HS, but recovered in HSRE and these changes were not associated with differences in caspase-3, -8 or -9 activity in any group. Protein abundance of apoptosis repressor with caspase-recruitment domain (ARC), cytosolic EndoG, as well as cytosolic and nuclear apoptosis inducing factor (AIF) were lower in muscle from old rats, and there was no age-related difference in the response to atrophy or regrowth. Soleus muscles from old rats had a higher number of ED2 positive macrophages in all groups and these decreased with HS, but recovered in HSRE in the old, while no changes were observed in the young. Pro-inflammatory cytokines in serum did not show a differential response with age to different loading conditions. Results indicate that at the measured time point the impaired skeletal muscle regrowth after atrophy in aged animals is not associated with a general lack of responsiveness to changes in loading conditions.
Keywords: Apoptosis; Autophagy; Hind limb suspension; Inflammation; Protein degradation; Protein synthesis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures

Similar articles
-
Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle.J Appl Physiol (1985). 2004 Jan;96(1):398-404. doi: 10.1152/japplphysiol.00454.2003. Epub 2003 Sep 26. J Appl Physiol (1985). 2004. PMID: 14514701
-
Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent.Am J Physiol Endocrinol Metab. 2003 Feb;284(2):E340-50. doi: 10.1152/ajpendo.00253.2002. Epub 2002 Oct 22. Am J Physiol Endocrinol Metab. 2003. PMID: 12397024
-
Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats.Appl Physiol Nutr Metab. 2010 Jun;35(3):310-8. doi: 10.1139/H10-022. Appl Physiol Nutr Metab. 2010. PMID: 20555375
-
Skeletal muscle hypertrophy and atrophy signaling pathways.Int J Biochem Cell Biol. 2005 Oct;37(10):1974-84. doi: 10.1016/j.biocel.2005.04.018. Int J Biochem Cell Biol. 2005. PMID: 16087388 Review.
-
Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy.Cells. 2020 Aug 26;9(9):1970. doi: 10.3390/cells9091970. Cells. 2020. PMID: 32858949 Free PMC article. Review.
Cited by
-
Alterations of macrophage and neutrophil content in skeletal muscle of aged versus young mice.Muscle Nerve. 2021 Apr;63(4):600-607. doi: 10.1002/mus.27158. Epub 2021 Jan 13. Muscle Nerve. 2021. PMID: 33386611 Free PMC article.
-
Soft tissue manipulation enhances recovery of muscle mass in a disuse model of sarcopenia.J Osteopath Med. 2025 Mar 13:10.1515/jom-2024-0247. doi: 10.1515/jom-2024-0247. Online ahead of print. J Osteopath Med. 2025. PMID: 40073288 Free PMC article.
-
Massage as a Mechanotherapy for Skeletal Muscle.Exerc Sport Sci Rev. 2021 Apr 1;49(2):107-114. doi: 10.1249/JES.0000000000000244. Exerc Sport Sci Rev. 2021. PMID: 33720912 Free PMC article.
-
Impaired proteostatic mechanisms other than decreased protein synthesis limit old skeletal muscle recovery after disuse atrophy.J Cachexia Sarcopenia Muscle. 2023 Oct;14(5):2076-2089. doi: 10.1002/jcsm.13285. Epub 2023 Jul 14. J Cachexia Sarcopenia Muscle. 2023. PMID: 37448295 Free PMC article.
-
Age-related responses to a bout of mechanotherapy in skeletal muscle of rats.J Appl Physiol (1985). 2019 Dec 1;127(6):1782-1791. doi: 10.1152/japplphysiol.00641.2019. Epub 2019 Oct 31. J Appl Physiol (1985). 2019. PMID: 31670600 Free PMC article.
References
-
- Rosenberg IH. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Am J Clin Nutr. 1989;50:1231–1233. - PubMed
-
- Bassey EJ, Fiatarone MA, O’Neill EA, Kelley M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci. 1992;82:321–327. - PubMed
-
- Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24:468–73. - PubMed
-
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–65. - PubMed
-
- Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the women’s health and aging study. Arch Phys Med Rehabil. 1999;80:130–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

